Although the concept (and claim) of anti-aging is frequently used in cosmetic research papers, market trends analyses and product advertisements, this term is not clearly defined and encompasses many ideas ranging from simple moisturizing, to age spot (lentigo) treatment, wrinkle reduction and skin-firming. These are curative-type approaches to anti-aging skin care. In contrast, preventative approaches claim to slow or stop symptoms of aging by protecting against free radicals and oxidants, enzymatic tissue degradation, inflammation, telomere shortening and glycation. Although curative and preventative approaches to aging appear to be very different, new research reveals they are closer than one might think. The same is true for the often-used distinction between intrinsic and extrinsic aging, which really cannot be separated from one other.
To quote Suresh Rattan:1
Biochemical and molecular [bases] of aging reside in the mechanisms of progressive failure of homeostasis or homeodynamics, which leads to the accumulation of damage in nucleic acids, proteins and lipids. This results in the impairment in functional ability at all levels of organization, thereby increasing the possibilities of a plethora of diseases and eventual death of the organism. Since homeostasis or homeodynamic ability of a living system is primarily due to its maintenance and repair processes, it is the progressive failure of maintenance and repair mechanisms that is the universal biochemical basis of aging and age-related diseases.2
In simpler terms, the moment humans begin to breathe and eat, “external” inputs of oxygen and sugar initiate lipoperoxidation and protein glycation, respectively. These are aging processes caused by free radical generation. Thus, to live is to age. So where and how can an anti-aging cosmetic ingredient be integrated? A few hints in the literature3-5 led the current authors to investigate certain peptides for their potential preventative and curative effects; highlights from the literature and the in vitro, ex vivo and clinical studies are described in the present article.
Mechanism Paradoxes
This article cannot present all the complex interactions between the phenomena of oxidative damage to DNA, proteins and lipids; mitochondrial energy production and tissue repair; and their observable macroscopic consequences. However, as noted, those processes relevant to the pursuit of developing a preventative and curative cosmetic active include the following.
Senescent cells: Rodier and Campisi6 reviewed cellular senescence and discussed apparent contradictions. Senescent cells release cytokines and matrix metalloproteinases (MMPs), which on one hand protect the body against tumor formation and help wound healing, but on the other hand contribute to chronic inflammation. One of the hallmarks of senescence resides in the establishment of the senescence-associated secretory phenotype. Senescence evolves in a multi-step process, which is defined by the quality of the secretion. The first step is the decision of whether to the start repair process or undergo senescence to stop the potential for forming a tumor. The steps after are characterized by the initiation and evolution of various protein secretions that can promote tissue repair, but also cancer progression, immune clearance and aging.
The paradox appears resolved when taking into account the age at which senescence occurs. In younger skin—i.e., in individuals of a reproductive age—both the autonomous, damage-induced cellular lapse into senescence and stimulation of wound-healing and tissue repair are mechanisms to defend against tumor formation and tissue degradation. However, in older skin—i.e., beyond the reproductive age—chronic, low level inflammation, e.g., IL-6 and IL-8 secretion, can have the opposite effect.
Similar contradictions appear in the process of wound healing, where inflammation and proteolysis are necessary prerequisites to tissue rebuilding via protein synthesis. Hsu et al.7 showed that healing proceeds in a temporally ordered fashion, with an increase in stress-induced senescent cells expressing higher amounts of transforming growth factor β1 (TGF-β1), collagen, fibronectin and MMPs than healthy cells in fibroblasts from wounded tissue sites. Here, the authors concluded that cellular senescence and turnover of the extracellular matrix (ECM) regulated by TGF-β1 in fibroblasts are critical for healing.
Progerin: The matter becomes further complicated when one tries to understand recent work on progeria, also called Hutchinson-Gilford progeria syndrome (HGPS).8 This condition is caused by truncated prelamin A (progerin), a major structural component of the lamina in the nuclear membrane, and is a rare genetic disease that accelerates aging, often at dramatic rates.9 Both keratinocytes and fibroblasts affected by progeria syndrome have abnormal nuclear morphology but despite morphological alterations in keratinocyte nuclei, the expression of progerin in the epidermis does not affect wound healing capacity, which incriminates other cells such as fibroblasts as being responsible for the cutaneous signs related to progeria syndrome.10
The mechanisms through which progerin causes tissue-specific abnormalities is not clear. In gene expression studies of progeria-affected dermal fibroblasts, several genes involved in building and maintaining the ECM showed altered gene expression, including collagens and MMPs. Additional reports describe the altered expression of elastin, laminins, type IV collagen and fibronectin as well as several proteoglycans.11 McClintock et al.12 have shown progerin-positive fibroblasts from unaffected individuals localized near the basement membrane and in the papillary dermis of young adult skin. In elderly skin, the number of these fibroblasts increases, and their distribution reaches the deep reticular dermis. Progressive telomere damage—another important factor in the aging process—plays a causative role in activating progerin production during the cellular senescence of normal human fibroblasts.13 Shortened telomere lengths are also associated with various skin pathologies.14 Accelerated aging syndrome studies can thus give a good image of the biological events related to normal aging. Nevertheless, it is still difficult to define how progerin, DNA damage, signaling proteins and the ECM are interconnected.15
Recent Approaches
Where do these contradictions leave the cosmetic scientist in the quest to reduce aging processes in skin? Repairing tissue by delaying senescence with antioxidants and anti-inflammatories has been investigated, especially in the protein synthesis of fibronectin, collagens and hyaluronic acid (HA), etc. Also, at high concentrations, the “copper peptide” (Gly-His-Lys.Cu2+) has been found to stimulate collagen synthesis.16 However, copper also has been found to induce premature senescence in human fibroblasts, even at subtoxic concentrations,17 along with higher TGF-β1 levels, cell enlargement and SA-β-galactosidase (SA-β-gal) activity. A palmitoylated (Pal-GHK) and biotinoylated (Biot-GHK) version of the same copper peptide at one-tenth the use level has been shown to enhance wound repair proteins in vitro, ex vivo and in vivo,18 and to increase cell proliferation (K67 mitosis rate) ex vivo.19, 20
Klusha et al.21 describe experiments where an immunoglobulin fragment (Gly-Gln-Pro-Arg) was found to be a potent inhibitor of stress signals. In relation, Mondon et al.22 showed that a palmitoylated form of the same fragment, Pal-GQPR, imparts anti-inflammatory activity by reducing the secretion of IL-6 and IL-8 both initially and after UV irradiation. Other ingredients have been shown to reduce senescence-associated SA-β-gal and increase collagen synthesis;23 to reduce progerin;24 and to reduce telomere shortening;25, 26 although these results were derived from single-parameter experiments. Of interest to the current investigation is a combination of Pal-GHK and Pal-GQPR peptides a, which was assessed in vitro for its effects on collagen, fibronectin and HA synthesis, as well as clinically for tissue repair activity.27
Materials and Methods
Peptides: Pal-GHK and Pal-GQPR were synthesized by a solid phase method, purified to > 95%, and characterized by high performance liquid chromatography (HPLC) and mass spectroscopy. These were dissolved in dimethyl sulfoxide (DMSO) at concentrations of 5,000 ppm Pal-GHK and 2,500 ppm Pal-GQPR, from which aliquots were drawn for in vitro experiments. The experiments were run at either 3.0 ppm of Pal-GHK + 1.5 ppm of Pal-GQPR, referred to hereafter as PEP 3%; or at 5.0 ppm of Pal-GHK + 2.5 ppm of Pal-GQPR, equivalent to PEP 5%.
Senescence: Human dermal fibroblasts were cultivated in Dulbecco’s modified Eagle’s medium (DMEM) in the presence of fetal calf serum (FCS) (10%) and 5% CO2 through several passages and reseeded after reaching confluence until cells stopped proliferating and entered replicative senescence. Three separate cultures were formed, in which the medium contained either no peptide and DMSO, as a control; PEP 3%; or PEP 5%. SA-β-gal activity was determined using fluorescein-di-β-galactopyranoside (FDG) as a substrate.28 The expression of progerin genes (Ct) was measured by quantitative PCR methods29 in the mRNA extracts of the senescent cells. Results were expressed as ratios of the mutated lamin A (progerin) gene in treated cells to untreated cells, weighted by the expression of housekeeping genes (β-actin).
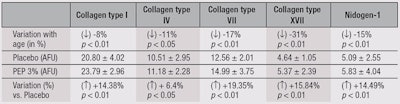
Ex vivo: Abdominal skin explants were obtained from 10 Caucasian women, ages 30 to 66 (mean = 49 ± 14 years) for studies of age-related changes in the dermal-epidermal junction (DEJ). Two groups of five members each were defined: 36 ± 6 years old and 61 ± 5 years old. For immunofluorescence, the skin explants were frozen in liquid nitrogen and kept at a temperature of -80°C until they were used. Immunostaining of collagens I, IV, VII and XVII, and nidogen-I was performed using specific monoclonal or polyclonal (coll IV and XVII) antibodies on frozen skin sections (7 µm). Nidogen-1, also called entactin, is a component of the basement membrane, alongside other components such as proteoglycans, laminin and fibronectin. Labeling was performed using secondary antibodies coupled with fluorescent dye. Labeling intensity was quantified by image analysis of full-picture mapping (20 pictures). The student’s t-test for non-paired values (two-tailed) was used to analyze results; p values < 0.05 were considered significant.
Clinical tests: Two randomized, vehicle-controlled clinical studies30 in 24 female (42-67 years old; mean = 56.1) and 39 male (40-64 years old; mean = 54.5) panelists were conducted to measure the wrinkle-reducing capabilities of test creams (see Formula 1) with or without PEP 3%. Note that only the 3% level of active was tested, due to efficacy and cost considerations. Volunteers had no history of allergy or skin lesions, and were not taking medications that could interfere with the study. In addition, subjects were not allowed to use cosmetic products that could interfere with the study—i.e., anti-wrinkle, reparative, restructuring or regenerating products—for at least four weeks prior. Following randomization, either the control or 3% PEP cream was applied to one side of the face in the crow’s feet area in a blind manner, twice daily for two months. The volunteers consented to avoid UV exposure throughout the duration of the study. The anti-aging effect was assessed using profilometry and image analysis, photography and cutometry (only for the female panel).
Another clinical study to determine the morphology and structure of the papillary dermis was conducted on 28 female volunteers with mature skin (51-72 years old). Women were selected based on prior echography examination of their skin to confirm a sufficiently large anechogenic area, i.e., not reflected by ultrasound, of the dermis and demonstrating a certain degree of aging. High resolution echography can be used to study the SLEB; this was discovered by de Rigal in 1989.31 Indeed, with age, SLEB increases in thickness and becomes less echogenic. This reflects a decrease in density of the thickest fibers, where changes in protein components—i.e., those observed histologically in 3D skin models—can be followed quantitatively. The breakdown and degradation of these proteins in the papillary dermis lead to a reduction in the echo signals recovered by the instruments.
Volunteers were required to follow a wash-out period using the test placebo for 15 days before measurements began. Subjects were required to make no change to their hormone status during the three months before and during the test; i.e., no change in contraceptive, replacement or curative hormone therapy. Again, subjects were allowed to use only the cosmetics provided during the study. Volunteers applied the 3% PEP cream (see Formula 1) daily for two months on the forearm and half-face, and the placebo cream on their opposite forearm and half-face.
In vivo reflectance confocal microscopy (RCM) and ultrasound analysis were performed. A high resolution 50 MHz ultrasound deviceb was used to evaluate the subepidermal low echogenic band (SLEB). Captured images from the device were analyzed, and the thickness and density of the SLEB were obtained. Note that to ensure accuracy, ultrasound results were expressed as the mean of five analyzed images. One hundred cine loop images, #1 through #100, were taken by moving the probe on a 3-cm site, and images #30, #40, #50, #60 and #70 were analyzed. This assumes to be a random analysis to represent the possible diversity of a site. The site also was accurately positioned between T0 and Tfinal, within a 1-mm error.
Analysis of the upper dermal fiber structures of the face was achieved using a reflectance confocal laser microscopec at 785 nm, also positioned accurately between T0 and Tfinal. Optical sections of the skin, 500 µm x 500 µm and parallel to the skin surface, were measured from the stratum corneum to the dermis (lateral/vertical resolutions: 1.25/5 µm) in 3-µm increments. Image analysis of the fibers was performed using software, and fiber fragmentation was calculated by measuring fiber perimeters. The student’s t-test for paired values (two-tailed) was used to verify results; p values < 0.05 were considered significant. The number of subjects (n = 28) and inclusion of a placebo in this study helped to ensure the accuracy of the results.
Results: Senescence
The combination of the two peptides at both 3% and 5% led to a marked (18.5% and 56.4%, respectively) and significant (p < 0.01, n = 4) reduction in the amount of SA-β-galactosidase detected in the senescent fibroblasts, compared with untreated control cells. Cells appeared to enter senescence later when incubated with the peptides, in a concentration-dependent manner. SA-β-gal, however, is only a biological marker of aging cells and senescence,32, 33 neither causing the latter nor interfering with it. Thus, to evaluate the peptide blend for a true anti-senescence effect, more specific mechanisms were tested.
Results: Progerin
Relatively recent discoveries34, 35 on the faulty processing of the lamin A protein, leading to the toxic progerin fragment, have stimulated extensive research into progerin and its relation to aging; including studies in the skin. The expression of progerin mRNA was henceforth studied in relation to the senescence-onset retarding peptide blend. Preliminary results demonstrated that senescent fibroblasts had approximately 2.2 times the progerin content as young fibroblasts. Thus, at the end of replicative senescence, total RNA of cells treated with 3% and 5% PEP or the control were extracted and subjected to reverse transcription. Progerin expression was studied using quantitative PCR on the cDNA with the help of specific triggers, inspired by the research of Rodriguez29 and Cao.13 The expression of progerin relative to normal lamin A, i.e., non-mutated and non-truncated, was measured to demonstrate that the progerin down-modulation in the presence of the peptides was specific and not due to modulation of all lamin forms. Abnormal HGPS fibroblast cDNA was used as the positive control. Figure 1 and Table 1 summarize the results, calculated from Equation 1:
R = Efficacy –(ΔCt product – ΔCt control) Eq. 1
where R refers to the ratio of progerin vs. β actin and normal lamin A vs. β actin (see Table 1); Efficacy = 1.95, per qPCR analysis apparatus manufacturer instructions; ΔCt product = Ct (target gene) product – Ct (HKP) product; and ΔCt control = Ct (target gene) control – Ct (HKP) control. HKP refers to the “housekeeping gene” or reference gene.
Treating fibroblasts in replicative senescence with the two peptides appeared to decrease progerin expression in a significant, dose-dependent manner. This diminished expression was specific to progerin because normal lamin A was not affected under the same conditions. Note that although progerin did not seem to directly affect protein synthesis, a decrease in collagen synthesis in senescent progeria fibroblasts was observed.36 In this context, an intriguing observation was made by Lewis,37 who showed that a small protein rich in proline and arginine (PRELP) is involved in the binding of collagens to basement membranes and cartilage. In relation, PRELP deficiency is connected to a number of symptoms of progerin-induced damage. It was therefore interesting to study the peptides described herein for possible activity at the DEJ level, to the degree that the Pal-GQPR sequence ends in –Pro–Arg (PR).
Results: Ex vivo
A test cream containing 3% PEP or the vehicle alone was applied to skin explants from young (36 ± 6 years old) and old (61 ± 5 years old) donors for five consecutive days. The explants were then examined histologically for changes in key elements of the DEJ and basement membrane, i.e., collagens I, IV, VII and XVII; and nidogen-1. This model allows the investigator to study the effects of aging and anti-aging ingredients on the papillary dermis—a thin layer below the epidermal/dermal junction. This part of the dermis is especially important for the maintenance of three-dimensional (3D) skin architecture. Age-related damage to the dermis, e.g., caused by UV-A, is clearly more destructive in the papillary layer than in the reticulate dermis.38 Table 2 summarizes the results. The differences in proteins between the two age groups were significant, as were the changes observed for skin treated with the peptides, in comparison with the vehicle cream.
Results: Echography
Progress in techniques for measuring and visualizing biophysical parameters in skin makes it possible to noninvasively confirm the results obtained in vitro and ex vivo on human volunteers. High resolution echography at 50 MHz (see Figure 2) was used to measure variations in the thickness and density of the SLEB on the inner side of the forearm, having little sun exposure, and on the more exposed external surface of the forearm. At the beginning of the study, the average SLEB thickness (n = 28) on the inner forearm sites was ≈ 175 μm (p < 0.01), and the outer forearm ≈194 μm (p < 0.01). The average SLEB density (i.e., grey level from grey scale images) was ≈ 19 on the inner forearms and ≈ 16 on the outer forearms.
Treatment of these sites with the vehicle cream did not change the thickness or density values significantly over one and two months (see Table 3). The application of the peptide cream, however, decreased the thickness of the SLEB by ≈ 10% and increased the density by ≈ 11%, with some panelists reaching values > 30%. Table 3 summarizes these values and shows the statistical significance of the results, in particular the progression with time from one to two months. What does this data mean? From the literature, datasets from approximately 400 volunteers of various ethnic origins enabled Querleux et al.39 to establish Equation 2:
y = 5.04 x - 62 Eq. 2
which expresses the thickness variation of the SLEB depending on age (see Figure 3). Using this equation and the present data, it is possible to translate the improvements of SLEB thickness obtained with the peptides into a theoretical decrease in aging of about five years.
Results: RCM
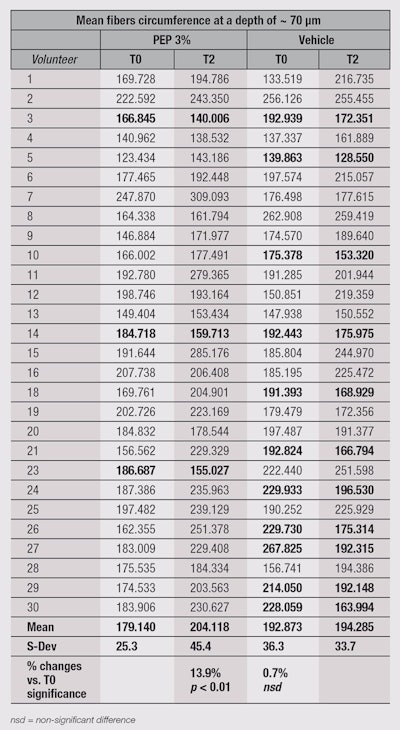
In the present study, RCM was used to investigate structural changes in the papillary dermis at the outer corner of the eye zone following two months of treatment with the test creams. The images obtained were analyzed to isolate and measure the size of fibers. Calculations of fiber perimeter were performed to evaluate fiber fragmentation. Indeed, this perimeter is longer when fibers are whole and not segmented.
Figure 4 and Figure 5, on Pages 50-51, show the results obtained after two months of treatment, clearly indicating a significant (p < 0.01) increase in fiber perimeter by approximately 14% and consequently a decrease in fiber fragmentation. The vehicle-only treated side saw no improvement of the fragmentation state of the fibers. The raw data obtained from this experiment (see on Page 52) shows that interestingly, not all panelists experienced improvements on the treated side of their face. Some even showed considerable increases in fiber perimeter on the vehicle-treated side—with or without corresponding effects on the contralateral side. This result is not surprising, given the experimental conditions, including an age spread of 51-72 years; and no control over lifestyles, compliance, the amount of product applied, time of massage, etc. However, there were only three cases where the peptide treatment recorded notably decreased fiber perimeter values, compared with 11 cases with notably increased fiber perimeter values. These numbers make the statistical analysis result not only significant in terms of p values, but also relevant.
Conclusion
The authors previously demonstrated the macroscopic benefits of a two-fold preventive and curative approach for anti-aging with the peptide combination described herein (PEP 3%)30—whose activity is now understood in a more detailed manner. Two vehicle-controlled, randomized clinical studies of the blend at 3% tested the wrinkle-reduction effects in 24 women and 39 men; results supported this duo approach. These activity of the peptides is based on various factors, including the anti-inflammatory activity of the Pal-GQPR peptide (reduction of IL-6 and IL-8), as well as the stimulation of collagen synthesis by the GHK peptide and its synergy with the tetrapeptide. Other factors include delaying the onset of senescence in cultured fibroblasts, concomitant with a reduction in progerin buildup; and repairing crucial DEJ proteins ex vivo, as shown clinically by echography (SLEB) and confocal laser microscopy (fiber fragmentation in the papillary dermis). Taken together, this approach constitutes a solid foundation for the claim made at the outset of the present article: that preventative and curative mechanisms are tightly connected and can be subtly influenced by the right choice of active ingredients, especially when they are based on peptides inherently present and active within the human organism.
References
- SIS Rattan, Principles of aging and the practice of anti-aging therapies, Asian J Exp Sci, 20 (1) 17-26 (2006)
- R Holliday, Understanding Aging, Cambridge, Cambridge University Press (1995)
- HR Choi et al, Stem cell recovering effect of copper-free GHK in skin, J Pept Sci 18 (11) 685–690 (2012)
- S Gallant et al, Carnosine as a potential anti-senescence drug, Biochemistry (Moscow) 65 (7) 866–868 (2000)
- LA Mullins et al, In vitro skin biomarker responses to a new anti-aging peptide, Pal-KT, J Am Acad Dermatol 60 (suppl) AB82 (2009)
- F Rodier and J Campisi, Four faces of cellular senescence, J Cell Biol 192, 547–556 (2011)
- WH Hsu et al, Cellular senescence occurring in the rabbit medial collateral ligament during healing, J Orthop Res 31 81–90 (2013)
- P Scaffidi and T Misteli, Lamin A-dependent misregulation of adult stem cells associated with accelerated aging, Nat Cell Biol 10 (4) 452–459 (2008)
- MA Merideth, LB Gordon and S Clauss, Phenotype and course of Hutchinson–Gilford progeria syndrome, N Engl J Med 358 (6) 592–604 (2008)
- Y Wang et al, Epidermal expression of the truncated prelamin A causing Hutchinson–Gilford progeria syndrome: Effects on keratinocytes, hair and skin, Human Mol Genetics 17, 2357–2369 (2008)
- IA Harten et al, Age-Dependent Loss of MMP-3 in Hutchinson–Gilford Progeria Syndrome, J Gerontol A Biol Sci Med Sci 66A (11) 1201–1207 (2011)
- D McClintock et al, The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin, PLoS ONE 2 (12) e1269 (2007)
- K Cao et al, Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts, J Clin Invest 121(7): 2833–2844 (2011)
- MG Kosmadaki and BA Gilchrest, The role of telomeres in skin aging/photoaging, Micron 35(3) 155–9 (2004)
- O Dreesen and CL Stewart, Accelerated aging syndromes, are they relevant to normal human aging? Aging 3 (9) 889–895 (2011)
- FX Maquart et al, Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl- L-lysine-Cu2+, FEBS Lett 238 (2) 343–6 (1988)
- L Matos, A Gouveia and H Almeida, Copper ability to induce premature senescence in human fibroblasts, Age (Dordr) 34 783–794 (2012)
- K Lintner and O Peschard, Biologically active peptides: From a laboratory bench curiosity to a functional skin care product, Int J Cosm Sci 22 207–218 (2000)
- K Lintner and P Mondon, Hair growth modulation, up or down: A review of ways to influence the biological events in the hair follicle, J Cosm Sci 61 (5) 397–398 (2010)
- V Arul, R Kartha and R Jayakumar, A therapeutic approach for diabetic wound healing using biotinylated GHK incorporated collagen matrices, Life Sci 80 (4) 275–84 (2007)
- VE Klusha et al, Neuroimmunoregulatory properties of short protein fragments in rats exposed to immobilization stress, Bull Eksp Biol Med 104 186–187 (1987)
- K Lintner, C Mas Chamberlin, P Mondon and O Peschard, IgG fragments regulate IL6 production in keratinocytes: Potential use in anti-age treatments, IFSCC 2005 Conference Proceedings 1-8 (2005)
- JT Bae et al, Protective effects of fermented citrus unshiu peel extract against ultraviolet-A-induced photoaging in human dermal fibrobolasts, Phytother Res 26, 1851–1856 (2012)
- C Verdy, JE Branka and N Mekideche, Quantitative assessment of lactate and progerin production in normal human cutaneous cells during normal aging: Effect of an Alaria esculenta extract, Int J Cosmet Sci 33 462–466 (2011)
- BR Zhou et al, Ginsenoside Rg1 protects human fibroblasts against psoralen- and UVA-induced premature senescence through a telomeric mechanism, Arch Dermatol Res 304 223–228 (2012)
- K Lintner, C Mas-Chamberlin, P Mondon and P Benech, Teprenone (Geranylgeranylacetone = GGA), a novel isoprene analog of geranylgeranylphosphate prolongs cell survival and improves skin condition, IFSCC 2007 Conference Proceedings 2.03 1–9 (2007)
- P Mondon et al, Evaluation of dermal ECM and EDJ modifications using four methods: Histology, MALDI-MSI, in vivo laser confocal microscopy and echography. Effect of age and peptide applications, IFSCC 2012 Congress Proceedings 359 37–38 (2012)
- NC Yang and M-L Hu, A fluorimetric method using fluorescein di-β-D-galactopyranoside for quantifying the senescence-associated β-galactosidase activity in human foreskin fibroblast Hs68 cells, Anal Biochem 325 337–343 (2004)
- S Rodriguez, F Coppede, H Sagelius and M Eriksson, Increased expression of the Hutchinson–Gilford progeria syndrome truncated lamin A transcript during cell aging, Eur J Human Genetics 17 928–937 (2009)
- US Pat App 2004132667, Compositions containing mixtures of tetrapeptides and tripeptides, K Lintner, Sederma SAS (Jul 8, 2004)
- J de Rigal et al, Assessment of aging of the human skin by in vivo ultrasonic imaging, J Invest Dermatol 5 621–625 (1989)
- F Debacq-Chainiaux, JD Erusalimsky, J Campisi and O Toussaint, Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo, Nat Protoc 4(12) 1798–806 (2009)
- K Itahana, J Campisi and GP Dimri, Methods to detect biomarkers of cellular senescence: The senescence-associated beta-galactosidase assay, Methods Mol Biol 371 21–31 (2007)
- JM Gonzalez et al, A-type lamins and Hutchinson-Gilford progeria syndrome: Pathogenesis and therapy, Front Biosci (Schol Ed) 3 1133–46 (2011)
- H Takeuchi and TM Rünger, Longwave UV light induces the aging-associated progerin, J Invest Dermatol 133 (7)1857–62 (2013)
- A Colige, B Nusgens and CHM Lapiere, Altered response of progeria fibroblasts to epidermal growth factor, J Cell Sci 100 649–655 (1991)
- M Lewis, PRELP Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: a new view of skin morphogenesis and aging, PLoS One 3(12) e4066 (2008)
- S. Mine et al, Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: A new view of skin morphogenesis and aging, PLoS One 3(12) e4066 (2008)
- B Querleux et al, Skin from various ethnic origins and aging: An in vivo cross-sectional multimodality imaging study, Skin Res Technol 15 306–313 (2009)