Sensory properties in skin care formulations are produced mainly by emollients, rheology modifiers, emulsifiers and humectants. Emollient esters are cosmetic ingredients that help maintain skin’s softness and plasticity, form semi-occlusive films for moisturizing benefits, reduce the itching sensation often present in dry skin, and improve the appearance of the stratum corneum. As components of cosmetic formulations, emollient esters act mainly as moisturizers, plasticizers and tactile modifiers when applied to skin.1, 2
In skin care emulsions, emollients are generally used at levels between 3–20% w/w, representing the second major ingredient after water. This use level varies depending upon several parameters including the oil phase composition, level of emulsifier blend, compatibility between ingredients, desired after feel, and the type, use level and solubility of UV filter in the ester (for sunscreens). Therefore, emollients play a major role in influencing the skin feel of formulations.3
Based on their chemical structures, emollients can be categorized as esters, hydrocarbons, glycerides, ethers, fatty alcohols and silicone derivatives. When formulating cosmetics, the product developer’s choice of emollient depends on several important factors such as chemical structure, polarity, molecular weight, spreading attributes, viscosity, solubility, contact angle and surface tension.4
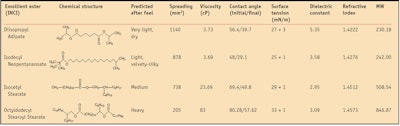
The present study examines four known cosmetic emollient esters in vitro to assesses their physicochemical properties and correlate them with in vivo sensorial performance. The emollient esters investigated include: diisopropyl adipatea, isodecyl neopentanoateb, isocetyl stearatec and octyldodecyl stearoyl stearated. These esters were selected for their broad range of molecular weights, with branched and/or linear-branched alkyl carbon chains. Each has a distinct chemical structure, physicochemical characteristics and sensorial profiles. For all in vitro and in vivo assessments, the esters were tested as pure materials and not incorporated into finished formulations.
Characteristics measured include spreading values on an artificial membrane, contact angle, surface tension, dielectric constant, refractive index and viscosity. Following in vitro assessments, an in vivo sensory panel test was conducted to investigate the perceived sensory attributes of the same emollient esters after application to skin. This study demonstrates that the physicochemical properties of ester molecules can be correlated with their distinct sensory profiles.
Spreading Values
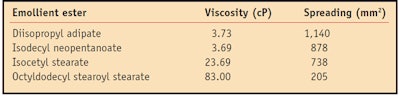
Spreading is the ability of a molecule to expand on a surface. This value changes based on molecular weight, viscosity and chemical structure.4 In general, molecules with low molecular weights and low viscosities have higher spreading characteristics. The spreadability of the four emollient esters was measured on a synthetic membrane that mimics the surface properties of human skin in terms of surface topography, pH, surface tension and wetting propertiese.5 The artificial membrane was cut into sections approximately 4 cm x 15 cm and hydrated in a small humidity chamber for 24 hr. The humidity was regulated by a solution of 15% w/w glycerin and 85% w/w water, which was placed at the bottom of the chamber. A 10-µL sample was placed with an electronic pipette onto the artificial membrane and the spreading area was measured after 10 min. Each emollient was measured in triplicate and repeated for a total of six measurements. The spreading values for all four molecules are shown in the Table 1.
Results indicated the spreading values for the tested esters decreased as follows: diisopropyl adipate > isodecyl neopentanoate > isocetyl stearate > octyldodecyl stearoyl stearate. Figure 1 illustrates the correlation between spreading values of the esters 10 min after application on the artificial membrane and their viscosities. As expected, as the viscosity of the esters increases, the spreading characteristics decrease.
Surface Tension
Surface tension is a property resulting from the attractive intermolecular forces within a molecule, such as dispersion, polar and hydrogen bonding.6, 7 Surface tension and contact angle can predict a molecule’s spreading characteristics.7 Generally, emollients with low surface tension spread easily while emollients with high surface tension are more difficult to spread.
The surface tension of the described esters was measured via the pendant drop method using a manual disposable syringe with a 1-mm diameter needlef. Surface tension values reported in Table 2 represent an average of 20 trials. The measurements were conducted at 21–23°C and 24–35% RH.
Diisopropyl adipate and isodecyl neopentanoate, the most polar esters analyzed here, demonstrated the lowest surface tension values when compared with all other esters. This is in agreement with their higher spreading values, lower molecular weights and lower viscosities. Isocetyl stearate exhibited a medium surface tension, correlating with its medium spreading value, viscosity and molecular weight. Octyldodecyl stearoyl stearate had the highest surface tension, also in agreement with its higher viscosity, higher molecular weight and lower spreading values, compared with the other esters.
Contact Angle
Contact angle is a measure of the wetting tendency of a liquid on a solid or another liquid, and is determined by the interactions between the three interfaces: liquid, gas and solid.8 Figure 2 is a schematic representation of the contact angle of a liquid droplet that wets or spreads on a solid surface at the air-solid-liquid contact point. In general, molecules with higher contact angles, i.e. θ > 90 degrees, do not wet a surface while molecules with lower contact angles, i.e. θ < 90 degrees, wet a surface.9, 10 Also, the smaller the contact angle of a molecule, the better its wetting properties.9
The contact angle calculation is based on Young’s equation:11
γSG = γSL + γLG cos θ
where γSG describes the solid-gas inter- facial energy, γLG describes the liquid-gas interfacial energy, and γSL represents the solid-liquid interfacial energy.
The contact angles of the tested esters were measured on a 0.02-in thick silicone substrate adhered to a glass slide to assure proper supportg. The measurements were conducted at 21–23°C and 24–35% RH. For this study, the static contact angle was measured, meaning the drop is produced before the measurement and kept at a constant volume (5±2 µL) during the measurement. Readings were taken automatically every 5 sec. Table 2 shows contact angle values representing an average of 10 trials, i.e. five trials on two different silicone sheets, for each ester. Images of the spreading droplet were captured with a high speed digital camera and analyzed by computer.
Results showed isodecyl neopentanoate and diisopropyl adipate had the lowest initial contact angle values, indicating these two esters wet the silicone substrate better than isocetyl stearate and octyldodecyl stearoyl stearate—the two more viscous esters. Initial and final contact angle values are reported in Table 2, along with the other physiochemical properties of the esters.
Further, diisopropyl adipate and isodecyl neopentanoate, esters with lower molecular weights and viscosities, also demonstrated enhanced spreading values on the artificial substrate, suggesting these two esters are more polar than the others investigated here. Isocetyl stearate, an ester with medium spreading, had a medium initial contact angle, 69.4 degrees, in good agreement with its medium viscosity, molecular weight and surface tension.
The most nonpolar ester investigated, octyldodecyl stearoyl stearate, had a higher initial contact angle value, 80 degrees, than the other esters; a higher surface tension (33 mN/m); lower spreading values on the artificial membrane (205 mm2/10 min); and a higher viscosity (83 cP). Therefore, this ester wet the silicone substrate less, suggesting good hydrophobic characteristics. The contact angle images for all four esters are shown in Figure 3, Figure 4, Figure 5 and Figure 6. The initial contact angle (θintial) was estimated at approximately 1 sec and the final contact angle (θfinal) was estimated at 5 min.
Viscosity
All esters are Newtonian, meaning their viscosities remain constant under applied stress. In the present study, viscosity measurements were taken using a viscometer with a small sample adapter, spindle 21 and sample chamber 13Rh. Samples were measured at speed 100 (shear rate 93 sec-1). The viscosity values (reported in cP) for all esters in the study are shown in Table 1 and Table 2.
Dielectric Constant
Dielectric constant relates to the polarity of a molecule.4 Matching the dielectric constants of emollient esters, and thus their polarity, is important in order to assure optimal solubilization and to improve formulation stability. The dielectric constants of the four esters were measured using a 10-KHz meterj. Measurements indicated that diisopropyl adipate had the highest dielectric constant (5.35 F/m), suggesting this ester is the most polar of all the esters analyzed in this study.
Refractive Index
Refractive index represents the ratio of the speed of light in a vacuum to the speed of light in the medium under consideration. The refractive index of an emollient can be related to the gloss or shine of the ingredient on skin or in a formulation; higher refractive indices result in more gloss or shine. Matching the refractive indices of emollients, oils, esters and glycols is important for achieving better clarity, compatibility and stability in cosmetic formulations.
Refractive index measurements of the four test esters were made using a refractometerk. The most polar esters, diisopropyl adipate and isodecyl neopentanoate, were found to have lower refractive indices than the other, more hydrophobic esters investigated here. Interestingly, the in vivo sensory evaluation described below reveals that the same polar esters were perceived as being less shiny than their nonpolar counterparts.
In vivo Sensory Evaluation
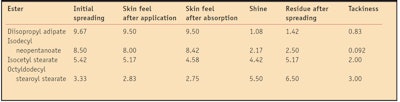
To correlate the in vitro physicochemical properties of the esters with their sensory profiles, a small in vivo sensory panel evaluation (n = 12) was conducted. Consistent with the in vitro investigations, the four emollient esters were applied neat to the skin; no control was used. One drop of each ester was placed onto the volar forearm of each panelist and spread into the skin until fully absorbed. Several parameters were evaluated, including initial spreading, skin feel directly after application, shine, residue after spreading, skin after-feel upon absorption and tackiness. These perceived sensorial characteristics were ranked from 1–10 by panelists (arbitrary units). Sensory values were averaged across all panelists.
The initial spreading parameter refers to the ease with which the ester absorbs upon application. Higher values indicate the ester spreads easily while lower values indicate the ester is difficult to spread. Skin feel directly after application relates to the initial sensorial perception in terms of how light, medium or heavy each ester was perceived. Therefore, a higher rating indicates the ester leaves a light and dry sensory perception on skin, while a lower rating indicates a heavier feel.
In terms of shine, higher values suggest an ester with increased shine while lower values indicate an ester with less shine. Perceived residue after spreading was evaluated as follows: higher values indicated more perceived residue while lower values indicated less residue. In terms of perceived skin feel after absorption, a higher rating refers to an ester that leaves a dry, light feel on skin, while a lower rating indicates a heavier, oily-feeling ester. For tackiness, higher ratings indicated a very tacky ester and lower ratings indicated an ester perceived as non-tacky.
The results obtained from the in vivo sensory evaluation are shown in Table 3. Overall, the less viscous, lower molecular weight esters felt less tacky and were perceived as leaving less residue on skin. Diisopropyl adipate was perceived as spreading the most, with the lightest, driest feel on skin, the least residue, the least gloss and felt non-tacky, relative to all other esters investigated. These results are in agreement with the in vitro measurements, which showed the same ester as demonstrating the highest spreading value on the artificial substrate. This is also in agreement with its lower values (relative to the other esters) for viscosity, refractive index, contact angle and surface tension.
Isodecyl neopentanoate was perceived as the second lightest ester while isocetyl stearate was ranked as a typical medium ester, in good agreement with all the in vitro measurements. Finally, octyldodecyl stearoyl stearate was perceived as the heaviest ester analyzed here, which correlates well with its physicochemical characteristics measured in vitro.
Conclusion
Based on in vitro measurements and in vivo sensory evaluation results, and correlating all the physical-chemical characteristics of the esters examined here, diisoproyl adipate was deemed a high spreading ester with spreading values in vitro > 1000 mm2/10 min. Meanwhile, isodecyl neopentanoate was rated a medium to high spreading ester, with spreading values in vitro between 950–750 mm2 /10 min; both diisoproyl adipate and isodecyl neopentanoate were found to impart a light, dry perceived after feel in vivo.
Isocetyl stearate was found to be a medium spreading ester, with spreading values in vitro between 750–450 mm2/ 10 min, and had a medium perceived after feel in vivo. Finally, octyldodecyl stearoyl stearate was the lowest spreading ester assessed, with spreading values between 450–50 mm2/10 min in vitro, andwith a heavy in vivo perceived after feel.
The approach of correlating in vivo perceived sensory attributes with in vitrophysicochemical properties can provide informative guidance for the cosmetic formulator in selecting specific emollient esters for various personal care and topical pharmaceutical formulations, and further predicting a product’s distinct sensory characteristics.
Acknowledgements: The authors want to thank David J. Moore, PhD, and Roger L. McMullen, PhD, for their editorial suggestions and support; Anna Gripp for her support to this project; and Vasile Gorcea for his help with graphic editing and illustrating support for this article.
References
Send e-mail to [email protected].
- M Gorcea, Efficient strategies for skin moisturization, NYSCC Cosmetiscope 14(2) 6, 7 (Feb 2008)
- A Rawlings et al, Moisturizer technology versus clinical performance, Dermatologic Therapy, 17 50–52 (2004)
- G Lanzendorfer, Lipids in skin care formulations, in Cosmetic Lipids and the Skin Barrier, T Forster, ed, Marcel Dekker, NY, Cosmetic Science and Technology series vol 24, Arch Derm 267–271 (2002)
- KF De Polo, Emollients, in A Short Textbook of Cosmetology, Verlag fur Chemische Industrie: H. Ziolkowsky KG, Germany 150–155 (1998)
- www.ims-usa.com (Accessed Nov 4, 2010)
- DJ Shaw, Liquid-gas and liquid- liquid Interfaces, Introduction to Colloid and Surface Chemistry, 4th edn, Butterworth-Heinemann: Philadelphia, USA 64–66 (1992)
- D Myers, Physical properties of surfactants, Surfactants in Cosmetics, 2nd edn, M Rieger and L Rhein, eds, CRC Press, Oxford, UK 52–54, 66–67 (1997)
- R Hunter, Thermodynamics of surfaces, in Foundations of Colloid Science vol 1 290–291 (1989)
- G Kumar and KN Prabhu, Review of non-reactive and reactive wetting of liquids on surfaces, Advances in Colloid and Interface Science, vol 133, Elsevier, Amsterdam 61–64 (2007)
- AW Adamson and AP Gast, Physical Chemistry of Surfaces, 6th edn, Wiley-Interscience, Hoboken, NJ USA 465–467 (1997)
- www.kruss.de/en/theory/measurements/contact-angle/introduction.html (accessed Oct 21, 2010)