The aim of antimicrobial efficacy testing (AET) performed during cosmetic product development is to predict microbial stability and consumer safety during use. The AET design includes referenced microbial strains and acceptance criteria. However, such tests do not include specific situations resulting from consumer use; for example, microbial flora encountered in normal environments; repeated insults; environmental condition variability; the impact of accessories and packaging; and the possibility of localized inoculation, e.g., via caps.
Therefore, it was deemed necessary to develop an additional test to strengthen the investigation. Here, the authors propose an approach to assess the microbial stability of a product during use, referred to as the Microbiological Use Test (MUT), and apply this analysis in a few case studies to predict the microbiological risk of commercial products. The described test has been used successfully in the development process of cosmetic products.
Experimental Design
The MUT test assesses, during the product development phase, the ability of a product to prevent its own microbial contamination during “standard” conditions of use. The aim is to perform a quantitative and qualitative assessment of potential contamination after a specified period of use. To ensure results, some parameters must be fixed; for example, blind testing, to ensure the product is used under conditions close to reality. Panelists should not be informed of the aim of the study—especially that the product will be microbiologically tested upon return. Also, packaging must be as close as possible to the final form, including being comprised of the same materials and utilizing the same closure system. This is a key point, as packaging plays an important role during product use and, therefore, in product contamination.
Timing and conditions also are important. Samples should be returned directly to the microbiological laboratory without extra manipulation prior to testing. The time between the last use and the first test must be fixed, e.g., 72 hr, to limit the recovery of transient microbes but allow for the detection of the more critical persistent contaminants. Further, it is necessary to have at least 20 samples involved to ensure a relevant assessment.
A general survey should be sent with each product to match the outcome with the user’s practices; also, directions for use, frequency and duration of application and specific requirements such as the use of an applicator should be specified. Finally, the number of subjects included in the study must be controlled, and they should be realistic potential user types who are physically located as close as possible to the laboratory.
Analysis Protocol and Other Considerations
Regarding sample analysis, the study must respect several steps. First, the product contamination bio-burden should be determined prior to study execution. Also, some samples should be retained in the laboratory at room temperature during the study execution and assayed two or three days prior to the return of consumer samples for validation; these results should be lower or equal to the initial count.
Besides MUT standards, the products must meet the usual microbiological specifications. Further, products, identified by lot number, require being assayed for preservative content. Regarding the study duration, the product must be used as often as possible; the design proposed here suggests at least three weeks.
Other considerations involve the design of the customer survey, directions for use and the study direction—i.e., start date, return date, etc. Product distribution to the selected panel of users and the return of samples must also be coordinated. As noted, it is necessary to manage the return date to ensure the delay between last use and first testing is within fixed limits, to be able to evaluate a possible regression of microbial count after a specified time.
Sampling Procedures
Testing should include all returned samples using methods proven suitable, such as ISO 21149, and following usual procedures applied in the laboratories. It is important to note that microbial contamination due to product use is typically not homogeneously distributed through the product. Therefore, sampling should occur as close as possible to normal use conditions. The sampling procedure must include the following steps:
- No mixing of the product before sampling;
- Surface sampling for liquid and semi-solid products;
- For products in tubes, retaining only the first expelled portion; and
- For products used with an applicator, sampling must use the applicator, which should be returned to its original place after sampling.
A second round of testing is then performed to assess whether the microbial contamination originally present was or was not eliminated after a certain period. This is a key element of product robustness assessment. It is recommended that this test be carried out six days after the last use; i.e., three days after the first testing, allowing for complete analysis within one week. This will not be feasible in all instances, and in certain cases, this could even alter the results and overall assessment. For example, with powder, a second sampling performed on a product scraped for the first testing will not be relevant since the entire contamination could have been removed. Therefore, when possible, it is also advisable to scrape only half of the surface for the first step, saving the second half for follow-up tests.
For products packed in tubes, the second dose will not always be representative, either. Therefore, it must be noted that the absence of recovered contaminant is not necessarily due to product performance; it may instead be driven by the absence of a homogeneous contamination. Finally, to ensure a good understanding of the results, it is necessary to assess other parameters such as organoleptic characteristics, physico-chemical parameters, preservative content and pH.
Assessment Specifics
Determination of the results is based on two criteria, described here. The first is the analysis for microbial contamination susceptibility; the second is the analysis for the product’s capability to reduce its original contamination after a short duration of time.
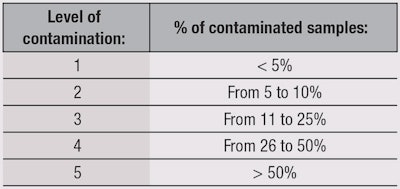
Susceptibility—first testing: The first test is for the product’s resistance to contamination, and is determined by only one criterion: the number of returned contaminated samples, independent from their levels of contamination. The presence of 10 cfu/g is enough to declare a sample as “positive.” Five levels were arbitrarily assigned by the authors (see Table 1) based on the % of positive returned samples. For example, Level 1 was assigned to the most robust products.
Reducing contamination—second testing: The second test to reduce contamination is performed six days after the last product use. During this period, products are stored in the laboratory at room temperature without exposure to sunlight. In this step, the criteria are different based on product characteristics; i.e., anhydrous vs. aqueous products.
Aqueous products are usually able to recover from contamination during the resting phase. Here, two levels have been set: A) bio-burden reduction, characterized by any significant reduction in count; and B) no bio-burden reduction. Considering the small number of samples involved (20), and taking into account the size of commercial productions, products will fail this test as soon as just one sample fails to reduce bio-burden.
In the case of anhydrous products, three levels have been set: A) bio-burden reduction, as described above; B) bio-burden stabilization, an intermediary level; and C) bio-burden increase, which, even if rare, must eventually be considered—especially for natural products.
MUT Assessments
The proposed MUT approach will result in products described as follows. First, each product will have a resistance level rating from 1 to 5 based on the first susceptibility testing. Then, based on the second testing, an additional rating will be assigned: A or B for aqueous products; A, B or C for anhydrous products.
The overall assessment will then be rated as:
IP (Insufficient Preservation): For products with no reduction, or an increase in, bio-burden;
S (Suitable): For products rated at levels between 3 and 5, and with either a bio-burden reduction or no increase with the second testing; or
RU (Robust during Use): For products rated at levels from 1 to 2, with either a bio-burden reduction or no increase as assessed with the second testing.
These product ratings are further outlined in Table 2 and Table 3.
Commercial Product Assessments
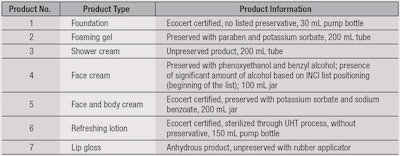
To test the proposed approach, seven commercial products were chosen based on their formula, function and use or non-use of a preservative system. The packaging, distribution mode and associated processes—and eventually, certification base, i.e., eco-labeling—of products were also taken into account. Specific choices were made to improve the feasibility and discriminant power of the MUT model. The chosen products and their basic information are included in Table 4.
Challenge tests following NF EN ISO 11930 were carried out1 on each product except number 7, the lip gloss. For products 1 through 5, criteria A were reached for bacteria, yeast and mold. For product 6, a refreshing lotion, no criteria were achieved; neither A nor B. The reason for this relates to packaging and is described later.
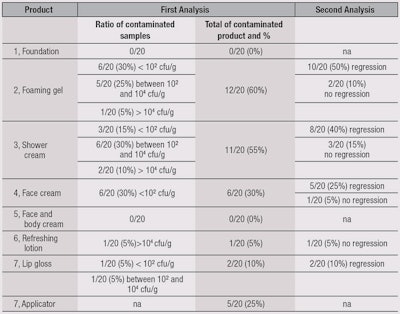
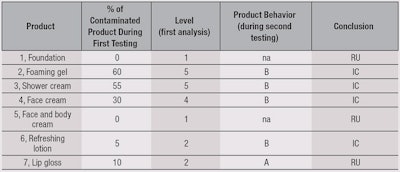
Results of the MUT assessment are summarized in Table 5. No contamination was found after the use of a face and body cream or a foundation (5). The two most contaminated products were the foaming gel (2) and shower cream (3). This is not surprising, as these are often stored in areas of risk due to the presence of water. The preserved shower gel did not provide better results, which is noteworthy.
For the face cream (4), the ratio of contamination was high (30%) but concentrations were low (< 10² cfu/g). Nevertheless, this low level of contamination may be associated with an absence of regression (5%). For the refreshing lotion, in only one panelist (10%) was a high level of contamination observed. In the absence of preservative system, no regression was noted.
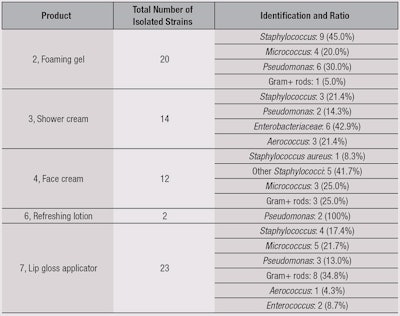
For anhydrous lip gloss, contaminations are isolated from the gloss itself as well as from the applicators. All the contaminants were isolated and identified, and results are summarized in Table 6. The authors noted a large proportion of cocci coming from the cutaneous flora, which are directly linked to use. Also observed was the fact that, among the recovered microorganisms, only one was specified: Staphylococcus aureus (1/22 Staphylococci recovered). A large quantity of Gram-positive bacilli were recovered as well. Another noticeable point was from the foaming gel (2), where the majority of recovered microorganisms were Staphylococci, while for the shower cream (3), Pseudomonas and Enterobacteriaceae were in majority.
Table 7 summarizes the final product assessments. Interestingly, none of the tested products were rated as Suitable (S). The face and body cream, foundation and lip gloss were classified as RU, whereas the face cream, foaming gel, shower cream and refreshing lotion were classified as IC.
Discussion: MUT vs. AET
All products evaluated in accordance with the ISO document (AET) except the refreshing lotion met the most stringent criteria. Nevertheless, following the MUT approach, some products would be classified as IC. This indicates that meeting the AET criteria is not sufficient to ensure a product will not become contaminated during use.
Also, as noted, the refreshing lotion did not meet either criteria A or B. In this case, protection of the ultra high temperature-sterilized formula (UHT) during use was provided by the pack. The protocol showed 5% of samples at most were contaminated (> 104 cfu/g) without any regression observed at the second analysis. This is the most important demonstration of the relevancy of the MUT to evaluate the global microbiological risk during use.
When a formula does not meet either criteria A or B, or where protection during use is based on packaging, then the MUT is capable of assessing the product’s robustness to prevent contamination. For satisfactory results (RU and S), this could become acceptable justification for product commercialization. The same reasoning may be followed for products where AET is not relevant, i.e., anhydrous lip gloss.
The difference in results between AET and MUT can derive from different elements. First, during the MUT assessment, contamination is iterative, whereas the AET assesses inoculation at one time. The MUT approach is more appropriate to assess formula robustness. Further, its contamination is natural and comes from the user’s environment, whereas the AET relies on simulated contamination with a limited panel. The likelihood of identifying preservation weakness to some contaminants is therefore increased using the MUT. Also, packaging is an important element to prevent microbial contamination during use, and only the MUT can assess this parameter since the AET only looks at the formulation independent of the packaging.
These various elements demonstrate why there is interest in this test. However, it does have limitations that must be highlighted. First, the MUT makes sense only if a second testing can be performed. Also, special attention must be paid to the sampling and re-sampling conditions to make sure the data is meaningful. When a product assessment is based on the second test, the outcome may be faulty, especially if contamination was diluted despite all precautionary measures taken during re-sampling. Finally, knowledge of the final package is critical to this test, indicating one must know the packaging and have it available at the time of testing. Since this often is not the case, this may delay testing to the latest development phases.
Conclusion
This study demonstrates, for seven selected products, the ability of the proposed MUT approach to determine a product’s robustness during use conditions. The specified criteria allows for a standardized investigation of the microbiological stability of products. Experimental results demonstrated that products tested three and six days after their last use can remain contaminated, as assessed via the recovery of specified microorganisms, potentially altering their organoleptic properties and product performance. This risk would not have been identified using only the current AET method, which seems not so predictive, with the exception of a product failing the test.
MUT appears to be an acceptable alternative to the AET. It additionally focuses on the element of microbial stability on the packaging materials required to prevent microbial ingress. Thus, microbial risk evaluation of the global product, i.e., formula plus packaging, during its normal use in an unprotected environment can be achieved using the proposed test design. This is of major interest, especially as the industry is developing more and more products with limited amounts of or no preservatives. Packaging then becomes a more significant part of a product’s preservation, and the MUT is able to assess packaging performance in situ.
References
- NF EN ISO 11930, Evaluation of the antimicrobial protection of a cosmetic product, available for purchase at www.iso.org/iso/catalogue_detail?csnumber=51037 (Jun 2012)