Hyaluronic acid (HA) is a naturally occurring polysaccharide frequently used as a functional ingredient in topical and subcutaneous anti-aging treatments. Unfortunately, in its native state, HA has certain characteristics that limit its value—i.e., it exhibits unusually rapid degradation and systemic removal, and it displays mechanical and structural weaknesses that limit its durability and performance.
Despite this, HA-based treatments are still the preferred choice for many in vivo applications such as dermal fillers since it is a natural component of the body and hence bio-compatible. In addition, its viscoelastic properties combined with a capacity to bind water increase volume and elasticity. Modifying the structure of HA to address its limitations is therefore an important strategy for enhancing and improving its value in cosmetic formulations.
Molecular weight (MW), distribution and structural features such as branching define the behavior of HA, and rheological characteristics quantify such behavior in terms of macroscopic properties of viscosity and viscoelasticity, which relate to the performance of HA in a formulation. This article first reviews the characteristics and structure of HA, then explores how multi-detector gel permeation/size exclusion chromatography (GPC/SEC) and microrheology techniques can be jointly applied to determine the structure-function relationships that support the successful inclusion of HA in cosmetic formulations. Experimental studies are provided to highlight the value of these techniques.
A Natural Biopolymer
A naturally occurring biopolymer, HA is one of the most hydrophilic molecules in nature.1 This functionality underpins its contribution to essential biological processes within the body and its value in cosmetic applications. As noted, HA is a preferred ingredient in many anti-aging creams and in treatments such as dermatological fillers, which leverage the polymer’s viscoelastic properties and capacity to bind water for effective soft tissue augmentation. When administered subcutaneously, HA builds a network within wrinkles and rhytides that draws in water to form gel-like structures and increases the elasticity of the skin, giving it a plumper, fuller appearance.
Naturally occurring HA has a short half-life of less than three days,1 so improving the durability of the polymer is essential to developing products with greater clinical persistence and an acceptable shelf-life. To do so, increasing both the MW and degree of cross-linking of the polymer has been shown to improve its mechanical strength and extend degradation times. However, these characteristics also impact the viscosity and viscoelasticity of HA. Thus, to formulate successfully with HA, it is essential to understand the relationships between molecular weight and structure, and rheological characteristics such as viscoelasticity, since these are directly related to product performance.
Analytical Strategies
Rheology is the study of material flow and can be used to quantify properties that directly impact consumer perception, such as spreadability and texture. While linking structural characteristics to product performance via rheological properties is fast and effective for formulation, this approach relies on an appropriately constructed analytical strategy.
Across industries, GPC/SEC is the technique of choice for determining the MW of polymers and macromolecules. This approach consists of two stages. The first involves the separation of a dissolved polymer sample on the basis of its elution volume, equivalent to hydrodynamic size, using a column containing microporous packing material. The second step is analyzing the eluting-sized fraction using one or more detectors.
Traditional GPC/SEC incorporates a single detector for concentration measurement. This is typically a refractive index (RI) or UV detector. With this set-up, a relative MW distribution is generated using a calibration curve correlating the elution volume to the MW of reference standards. If the MW to hydrodynamic size ratio of the sample differs from that of the standards, results will be inaccurate. In addition, single detector GPC/SEC offers no information about the structural characteristics of the polymer.
Modern GPC/SEC instruments incorporate additional detectors alongside RI to increase the informational productivity of each analytical experiment. Triple detection GPC/SEC, for example, typically adds a light-scattering detector and a viscometer. The light-scattering detector delivers absolute MW data, without external calibration, from measurements of the intensity at which a molecule scatters light. A viscometer can be used to access a number of viscosity parameters for the sample—especially intrinsic viscosity (IV). IV is an important characteristic related directly to the density of the polymer molecule, and is therefore helpful in determining structural information.
Each additional detector adds to the information gathered from each GPC/SEC experiment. However, beyond this, detectors can work synergistically. The following study shows how such synergies can be used to investigate the structural characteristics of different forms of HA.
Case Study 1: Multi-detection GPC/SEC
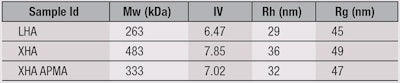
To investigate structural changes in HA, three samples were prepared for analysis by multi-detector GPC/SEC. The first was a native linear HA (LHA), which was used to form the baseline for comparative studies. The other two samples were modified via cross-linking and derivatization reactions.2 One, referred to here as XHA, was auto-cross-linked via ester bonds using carbodiimide chemistry. The other, XHA-APMA, was cross-linked in the presence of the nucleophilic agent N-(30Aminopropyl) methacrylamide hydrochloride (APMA) to encourage branching.
Table 1 summarizes the quantitative parameters obtained using GPC/SEC with triple detection—including RI and multi-angle light-scattering (MALS) detectors, and a viscometera. The absolute MW data provided by the MALS detector indicated the MW of the XHA cross-linked material was much higher than the LHA sample; the XHA-APMA sample had an intermediate MW. The MW of HA directly influences its response to mechanical stress, with higher MW having stiffer characteristics and greater elasticity. As a result, higher MW HA is typically the preferred option, for example, for intraocular applications requiring greater stiffness and longevity, whereas the lower MW material is more suitable for anti-aging products.
Constructing a Mark-Houwink (M-H) plot for the three samples provides detailed insight into their structure (see Figure 1). An M-H plot is a log-log graph of molecular weight versus intrinsic viscosity. Its y intercept provides information about the flexibility of the backbone, while the gradient is associated with the degree of branching. Therefore, a material with a high degree of branching tends to have a shallower slope than one with a more linear structure. The construction of an M-H plot relies on having MW and IV data, which underlines the value of triple detection.
Here, the linear structure of the LHA is reflected in the gradient of the M-H curve, which is close to 1 across the whole molecular weight range. In contrast, the gradient of the other samples becomes shallower as molecular weight increases. At any given molecular weight, most especially in the high molecular weight range, both the XHA and XHA-APMA have a lower IV than the LHA.
However, IV is inversely proportional to the molecular density of the sample, so these deviations toward lower IVs provide insight on impact of the modification reactions. The cross-linking in the XHA sample is responsible for its high molecular density. The fact that the IV of the XHA-APMA is higher than that of the XHA at any given molecular weight suggests this sample has a substantially lower degree of cross-linking. Reaction in the presence of APMA has favored the uptake of APMA on the substrate, inhibiting the cross-linking process.
In the lower MW region, the XHA-APMA and LHA plots overlay one another, but there is divergence in the higher MW region as a result of this branching. Clearly, multi-detector GPC/SEC is helpful in generating detailed insight into molecular structures. However, to use this information in formulation, understanding how MW and structure impact the rheological properties that define product performance is necessary.
Understanding Rheological Behavior
The rheology of a solution is governed by the volume of dispersed phase within it. For a polymer solution, this volume is influenced by the molecular weight of the polymer and its concentration. In solution, polymers such as HA take the form of expanded random coils. Figure 2 illustrates what occurs as the concentration of these coils is increased.
At low concentrations, the polymer coils have sufficient space to avoid interaction. In these dilute systems, the viscoelasticity of the sample is therefore governed by the properties of a single coil multiplied by the number of coils present. Thus, the properties of the coil itself and how the surrounding liquid flows through it determine behavior. This behavior can be described quite adequately by the Rouse-Zimm model, which considers a polymer chain in solution to be analogous to a chain of alternating beads and springs connected in series. Here, the viscosity results from the beads dragging in the solvent and elasticity results from stretching of the springs, simulating stretching of the polymer chain.3, 4
As the concentration of polymer coils increases, however, the coils become prone to entanglement since there is insufficient space for them to remain discrete. The concentration at which this happens is referred to as the critical overlap concentration, indicated by c*, which can be calculated either from measurements of the radius of gyration, Rg, which is a measure of the size of the polymer molecule; or from IV data.3
Above c*, the rheological behavior of the system is no longer governed by the properties of single coils, but rather by interactions between multiple coils. These interactions result in a sharp rise in viscosity and the onset of more substantial elasticity. The frequency or timescale over which shear is applied will define whether viscous or elastic behavior is dominant (see Figure 3 and Introduction to Viscoelasticity sidebar).
At low frequencies, Rouse-Zimm type behavior is observed, as in more dilute systems. However, at higher frequencies, a “rubbery plateau” region is established, where the elastic modulus becomes dominant over the viscous modulus. On these timescales, the polymer chains have insufficient time to free themselves from entanglements, causing tension in the chain segments between coupling points and hence elastic dominant behavior. The length of this plateau relates to the MW of the polymer and also its concentration. Further, at extremely high frequencies, polymer solutions undergo a phase transition to give a glass-like response equivalent to that usually seen below the glass-transition temperature, where polymer motion is highly restricted except for localized vibrational and rotational modes of deformation.
Quantifying viscoelasticity is an essential precursor to incorporating a polymer in a formulation in such a way as to confer consumer appeal. To do so, rotational rheometry is a standard technique used for oscillatory testing; see the Introduction to Viscoelasticity sidebar. However, this technique is limited in cases of exceedingly high frequency testing or weakly structured, dilute solutions due to inertial effects associated with both the sample and instrument. As an alternative, microrheology is emerging as an approach to address these issues and a technique based on this principle is described in more detail in the next case study.
Case Study 2: Microrheology
Microrheology is based on the principle that the movement of probe particles dispersed within a suspension or solution is related to the viscosity and viscoelasticity of the system. In relation, dynamic light scattering (DLS) is one technique used to track such movement of probe particles. Taken together, DLS microrheology can be successfully implemented with much smaller sample volumes than are typically needed for a mechanical rotational rheometer; it is also well-suited for polymer solution characterization.5
To compare the rheological performance of a cross-linked polymer with linear polymers of higher and lower MW, the LHA and XHA samples produced for Case Study 1, 5 mg/mL of each of these samples were analyzed, along with a high molecular weight linear sample, HHA, using DLS microrheologyb. Figure 4 shows the resulting flow profiles; here, a table is included showing MW, IV and Rg data measured during the GPC/SEC experiments, along with the corresponding calculated values of c*.
At 5 mg/mL, the concentrations of the tested solutions were below the c* for XHA, which had the highest c* of the three samples. The flow profile for XHA showed that correspondingly, it exhibited the most dilute solution behavior. As the Introduction to Viscoelasticity sidebar explains, the G′′ was in fact dominant at all frequencies, meaning the solutions would flow and have limited elasticity.
The LHA had a lower c*, much closer to the test concentration. For this solution, the G′ was much higher than for XHA, and at higher frequencies, G′ and G′′ became close and parallel with a gradient of 2/3 when plotted logarithmically. The shape of this profile is associated with classical Rouse-Zimm dilute solution behavior.
In contrast, the profile for HHA, which had the lowest c*, showed clear evidence of a “rubbery plateau,” i.e., a region where G′ exceeds G′′. This behavior is associated with the chain entanglement that occurs at polymer concentrations above c*. Further testing of HHA at a concentration of 3 mg/mL (data not shown) suggestedthat diluting the sample to this extent established Rouse-Zimm dilute solution behavior, as was observed with LHA.
Figure 5 shows the complex viscosity as a function of frequency profile for each of the HA samples, extracted from the DLS microrheology data. For polymer solutions such as these, profiles of complex viscosity as a function of frequency mirror standard shear viscosity profiles, as typically measured using a rotational rheometer.6 The results show all three samples are highly shear-thinning and higher molecular weight samples have higher viscosity. An interesting observation is that the XHA appeared to exhibit a viscosity plateau at higher frequencies. This is attributed to the cross-linking in this sample, which physically inhibits the movement of polymer chains relative to one another.
Conclusions
To formulate successfully and efficiently with polymeric ingredients such as HA, understanding the correlation between MW and performance-defining rheological properties such as viscosity and viscoelasticity is essential. The case study data presented here demonstrates how multi-detector GPC/SEC and DLS microrheology can be applied to generate the required information and adopt a knowledge-led approach to cosmetic formulation.
References
- FS Brandt and A Cazzaniga, Hyaluronic acid gel fillers in the management of facial aging, Clinical Interventions in Aging 3(1) 153-159 (Mar 2008)
- www.researchgate.net/publication/251509148_Comparative_analysis_of_commercial_dermal_fillers_based_on_crosslinked_hyaluronan_Physical_characterization_and_in_vitro_enzymatic_degradation (Accessed Apr 16, 2015)
- www.malvern.com/en/support/events-and-training/webinars/W140625ScienceOfBeauty.aspx (Accessed Apr 16, 2015)
- RG Larson, The Structure and Rheology of Complex Fluids, Oxford University Press, New York (1999)
- www.malvern.com/en/support/resource-center/Whitepapers/WP120917IntroDLSMicro.aspx (Accessed Apr 16, 2015)
- www.malvern.com/en/support/events-and-training/webinars/W130320Cox-MerzRule.aspx (Accessed Apr 16, 2015)