Protecting colored hair is a topic that appeals to consumers almost all over the world since, although artificial colors are supposed to be permanent, they always eventually fade. As a result, according to Mintel’s product database,1 of the nearly 16,000 water-based shampoos launched between January 2007 and June 2012, approximately 3,200 of them mention color or fading in their descriptions. Many of these shampoos focus particularly on the color fade that occurs after coloration, such as by shampooing and exposure to ultraviolet (UV) radiation from the sun. These factors are known to cause color fade, although not to the same extent.
Over the past five years, increasing numbers of consumers dyeing their hair have led to a steady increase in shampoos claiming color protection—and other than just “anti-UV.” In Europe, for instance, “anti-washout” shampoo launches represented 13.5% of the shampoo market in 2007, and increased to 21% in 2011. In North America, the same trend was observed; 22% of shampoos launched in 2007 made “anti-washout” claims, which increased to 32% in 2011. In the meantime, true “anti-UV” offerings remained constant at best in both regions, if not declined. This is with reason: The color protection segment is now far more concerned with shampoos claiming protection against color washout rather than UV.
As a matter of fact, water itself, as will be illustrated, significantly contributes to color fading caused by washout. The use of surfactants, i.e., anionic, amphoteric, neutral, is not without effects as well.2, 3 Thus, formulators might choose different options to create anti-washout shampoos, structured or micellar. For structured shampoos, formulations based on branched anionics like sodium trideceth sulphate (STDES) can be designed to help reduce fading.2 For micellar ones, formulators will favor amphoteric surfactants like amphoacetates, reputedly milder than cocamidopropylbetaine; and when they want a “sulfate-free” version, they will almost always turn to alkylpolyglucosides (APGs).2, 3
Of the 3,200 color-fade shampoos launched, referenced above, only about 900 actually relate to UV or sun protection. The remainder, and hence the majority, necessarily correspond to the fading caused by exposure to water and surfactants during shampooing. These two product categories are clearly technically distinct and rely on very different formulation habits. Thus, primary anionic surfactants, secondary amphoteric and nonionic surfactants, cationic polymers and silicone oils all significantly differ between the two.
Besides UV and washout, heat exposure after coloring, i.e., the use of a blow dryer—a time-saving habit most consumers would not sacrifice, also has become a hot topic as a potential source of hair damage that measurably impacts color durability. “Heat-protecting” shampoos represented 6% of the total shampoo market in Europe in 2007, which increased to 8% in 2011.1 This category has become very active in the past few years. Temperature affects hair structure in part due to its effects on the speed with which water leaves the hair shaft. When wet hair is exposed to sufficient heat, for instance when hot-ironed, the water trapped under the cuticle cells can make hair bubble4 since molecules rapidly vaporize but cannot leave the porous structure quickly enough.5 Since the heat controls how rapidly water leaves hair, it can damage not only hair’s physical structure, but also artificial dyes. Heat is thus likely to lead to accelerated color fade.
In the same way shampooing, UV and heat affect hair after coloration, damage caused before coloration also significantly alters color durability. In particular, perming and bleaching are routinely used by consumers and hairdressers to shape hair in different ways or remove original color to prepare for artificial coloring. Hair in its virgin form is covered with fatty acids that make it hydrophobic.6 Bleach is used to lighten hair by dissolving its melanin pigments7 through a well-known irreversible oxidation process.8 In doing so, bleach also removes surface fatty acids, making hair hydrophilic very early in the process.9 Meanwhile, hair becomes more porous and hence is prone to absorbing water—a phenomenon consumers have observed as increased time and heat required to dry bleached hair.
Perms, on the other hand, change the shape of hair and increase or decrease volume depending on the consumer’s preference. A perm reduces hair’s disulfide bonds,10 a side effect of bleaching as well,11 which are then reformed in new positions by mild oxidation. Perms have little impact on the hair surface as far as wettability is concerned, since changes in hydrophobicity are hardly measurable. Both chemical treatments measurably reduce hair strength and resistance to breakage.12 While perms should not be used repeatedly, bleach, which is part of the coloring process, can be carried out as often as every six to eight weeks to cover hair roots. As hair grows about 1 cm per month, a 30-cm hair may be permed twice and bleached (even mildly) more than 10 times in the course of its growth.
With so many different sources for damage before and after coloration, shampoos dedicated to color protection are often shouldered with a lot to repair and prevent. As a result, they must be carefully designed. It is therefore necessary to know how much each source of damage really impacts color durability, and with which source formulators should most be concerned. The present article assesses the impact of combined hair damage types on color fade and considers formulation aspects to improve color.
Materials and Methods
Tests were conducted on different types of hair damaged before and after coloration in different ways, i.e., by bleaching, perming and exposure to UV and heat, as well as combinations of these. To assess the durability of artificial color on these different types of hair, repetitive shampooing was always performed on three different tresses, to ensure reproducibility of the results. This shampooing, conducted by expert technicians, could be carried out with either: water alone; a model, simplified surfactant base, suitable for shampoos; a model surfactant base with added silicones, to assess their ability to improve color retention; or a commercial color protection shampoo, used as a reference.
Test hair: Caucasian hair, virgin or chemically damaged by single or double bleaching, was used for the described studies.a Swatches weighed 4 g, and were 17 cm long and 2.5 cm wide. Prior to any treatment, each tress was cleaned with 3 mL of a 10% w/w active sodium laureth sulfateb solution in water (see Formula A, Table 1), shampooed for one minute, and rinsed with tap water for another minute. In cases where damage was desired, virgin tresses were carefully bleached and/or permed with commercial productsc, d following the instructions precisely as consumers would, and waiting several days between bleaching and perming when the two were combined.
Colored hair: Tresses were dyed with commercial coloring kits, again following instructions as consumers would. Coloration duration was sometimes varied for a given purpose, i.e., from 35 to 50 min, depending on the intensity of the shade desired, with different types of damaged hair. Several shades were investigated, including auburn, ginger and ruby but for the reader’s sake, only investigations on auburn are described here;e note that similar results were obtained with the other colors.
Post-color weathering: Technicians waited 48 hr after coloration, as hairdressers typically advise, before imposing post-color damage of the following types.
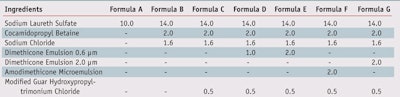
Shampooing: A commercial color-protecting shampoof and several test shampoos were used on colored hair. For each 4 g-tress, 0.8 g of shampoo was applied, massaged for 45 sec, and rinsed for another 45 sec. All test shampoos were based on a conventional chassis representative of the hair care segment (see Formula B in Table 1), comprising: 14% w/w active sodium laureth sulfate, 2% w/w active cocamidopropylbetaineg and 1.6% w/w active sodium chloride.
To this model chassis were added several types of silcones at 1% or 2% w/w active silicone, coming either from conventional dimethicone emulsions of different diameters, i.e., 0.6 µm and 2 µmh; or from a cationic amodimethicone microemulsion j. These oils were introduced together with 0.5% w/w active of a prototype polymer developed for color protection-a modified guar hydroxypropyltrimonium chloride.13
UV exposure: Repeated UV exposures were carried out with a xenon arc lampk at 500 mW/m² between 300 nm and 800 nm for 8 hr at a time, up to five times—with a 16-hr rest between exposures.
Heat exposure: After a shampoo cycle, the tresses were dried in a ventilated ovenm. The drying temperature and duration were usually 45°C for 1 hr, but these conditions also were varied from 30°C to 75°C, up to 75 min.
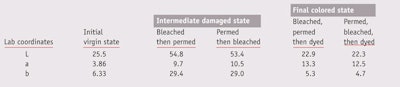
Color characterization: The color of each tress was determined before and after any chemical treatment. These colors, in the form of L*a*b* coordinates, were measuredn at 10 different points along the three different tresses, to ensure reproducibility. Recorded were: the initial color before dyeing (Linitial, ainitial, binitial), whether this tress was virgin, bleached and/or permed; the color after dyeing (Ldyed, adyed, bdyed); and the color once faded by repetitive degradations (Lfaded, afaded, bfaded), whether by means of shampooing, or exposure to UV or heat. In practice, technicians clamped down each tress in its dry state, at room temperature, on a black mate plastic board. The tresses used were dense enough to perfectly cover the color of the substrate. As noted, the tress’s color was measured at 10 different points along its length, in the central part of a given tress, and each assessment was repeated on three tresses, for a total of 30 measurements for each trial.
Equation 1 defines how much the tress color has changed from the initial state to the state after coloration; in other words, how much color has been successfully incorporated.
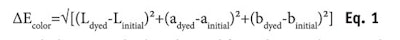
On the other hand, Equation 2 defines how much color is lost upon weathering. The ratio ∆Efading/∆Ecolor is a relative measurement (in %) of how much color resists this weathering. In several cases, this quantity turned out to be more appropriate than the absolute one, ∆Efading, simply because depending on the pre-color damage and the shade chosen to dye hair, the color obtained was different. To make the data comparable, normalization such as this is necessary.
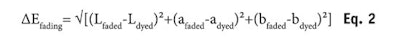
Results and Discussion
Pre-color damage: In practice, whether a tress has been bleached then permed or vice versa, and then dyed, the final artificial color achieved was nearly the same (see Table 2). This gives the illusion that the order in which these chemical treatments are carried out does not really impact the final color. Unfortunately, once the hair is shampooed, the differences rapidly become visible. As Figure 1 shows, when bleaching is carried out before perming and coloring, 50% of the color is lost after five shampoos, in comparison with only 30% when the perm is done first. The order between perm and bleach therefore has a considerable impact on color retention.
From a scientific standpoint, the extra porosity resulting from bleaching may allow the perm to diffuse faster and further, breaking more disulfide bonds than it normally would. This significance of the order in which damaging treatments are carried out somewhat explains why hairdressers advise to wait 48 hr between them, if consumers want their hair to be colored in an optimal and durable way. This “sort of” practical experiment also evidences how detrimental to color duration the accumulation of repetitive damaging pre-treatments can be.
Post-color—shampoo and UV damage: To determine whether shampooing or UV is the main factor behind color fade, the effects of each were quantified. More or less damaged hair, i.e., singly or doubly bleached, were dyed simultaneously and either: shampooed with the commercial “color protection” shampoo, exposed to UV, or both shampooed and exposed to UV; up to five times in each case.
For formulators, protection against UV from a rinse-off shampoo imposes the efficient delivery of an anti-UV active onto hair, and apart from benzophenones-4 and -5, all other actives are usually not water-soluble. Ethylhexylmethoxycinnamate, benzyl salicylate and titanium dioxide are dispersible as particulate agents, while benzophenone-3 and butyl methoxydibenzoylmethane are both oil-soluble. The efficient delivery of such insoluble actives then relies on polymeric cationic agents, historically used in 2-in-1 shampoos to deliver insoluble silicone oils. It is therefore no surprise to find cationic guars, reputedly good at delivering insoluble actives, in more than half of the “anti-UV” shampoos launched.
With dyed, single-bleached tresses (see Figure 2, top), combining UV with shampooing resulted in larger absolute fading than either alone. This result is consistent with previous findings and was true regardless of the shade used.14 The impact of shampoo and UV separately on color fading was comparable and in fact, almost additive. This implies that consumers with only mildly damaged hair may rely equally on “anti-UV” or “anti-washout” products to protect their colored hair from both types of damage. However, with dyed double-bleached tresses (see Figure 2, bottom), the impact of UV alone was negligible, with only a slight additive effect over shampooing. On hair heavily damaged by repeated bleaching, shampooing was the largest cause of fading. Thus, consumers should be more worried with their shampooing habits and exposure to water itself rather than everyday exposure to the sun.
Post-color—heat damage: An oven set to different temperatures to dry colored hair between shampoo cycles can be used to determine the relative impact of drying, both in duration and temperature. As expected, longer drying times and higher temperatures increased fading (see Figure 3). Heat (even reasonable) as well as a prolonged exposure to heat measurably aggravated fading. Figure 4 compares the fading of auburn tresses washed either: 10 times in a row without ever drying them, i.e., “continuous” washing; or auburn tresses shampooed and dried at 45°C for 2 hr between shampoos up to 10 times, i.e., “sequenced” washing. Doing so simulated the level of fading consumers would face if they never dried their hair between shampoos, in comparison with normal conditions of use.
For the sake of completeness, shampooing was then carried out either with water or with the model surfactant bases. Although this simple experiment might not seem realistic, since hair will dry eventually, it revealed that when shampooing is followed by drying, the extent of fading after 10 shampoo/drying cycles is much larger (∆Efading = 7 to 8 ± 0.5) than when tresses are simply shampooed 10 times in a row without ever being dried (∆Efading= 4 to 5 ± 0.5). This is true whether hair has been shampooed with a surfactant base or simply exposed to water. This shows that the very habit of repeated shampooing and drying hair every day significantly aggravates fading.
It is believed that physically, as hair absorbs water, swells upon shampooing, then shrinks upon drying, a fatigue phenomenon may be responsible for this aggravation of color fading. Heat is well-known to have an impact on the physical properties of hair itself, like aging or degradation,15 but these findings also suggest that heat will have a direct impact on the retention of the artificial colors introduced inside hair. Extra care should therefore be taken with artificially colored hair to prevent it from fading more than it normally would.
Formulation Impact and Silicone Addition
As mentioned previously, the formulation itself—and more precisely, the choice of the surfactants and additives, can help design better “anti-washout” color-protecting shampoos. In this category, sulfate-free formulations are very prominent; they represented three or four launches out of 10 over the 2007–2011 period in Europe and North America. Although little proof has been published on the color protection benefits of sulfate-free shampoos, the belief that sulfates are detrimental to color retention is strong. Thus, formulators might choose to develop formulas consistent with this belief. However, sulfate-free shampoos—and their associated claims—remain a niche in the shampoo market, not only due to the difficulty in achieving technical requirements like thickening and hair conditioning, but also (and mostly) because of cost. Therefore, formulators in many cases instead turn to classic sulfated shampoos.
With water being a large source of fading, formulators understand how necessary it is to form a chemical barrier on the hair surface to prevent the penetration of water. This is precisely what silicones like alkymethicones or amodimethicones do in 2-in-1 sulfated shampoos. They already play this role in the coloring step, as they often are added to coloring kits.16 Also, their delivery onto hair has been optimized in 2-in-1 shampoos, historically based on sulfates such as SLES.
As far as color maintenance is concerned, delivering a silicone onto hair will restore its hydrophobicity, which should help to some extent protect artificial color from fading. For this purpose, several neutral or cationic dimethicones, PEG- or alkyl-modified silicones, are available commercially. The most commonly used is conventional insoluble dimethicone, which is generally dispersed as an emulsion in 2-in-1 shampoos. However, as is illustrated here, the choice of silicone for color-protecting shampoos is paramount.
Double-bleached hair tresses, colored auburn with the same commercial kit described, were shampooed up to 10 times with the model shampoos based on different silicones (see Table 2). Figure 5 shows that adding a silicone to a shampoo containing a guar able to flocculate and deposit silicone in an optimal way resulted in improved color protection, which is consistent with published data.17 The performance, however, depended on the size, type and quantity of silicone added: 2% w/w of a silicone emulsion of diameter 0.6 µm performed better than 1% w/w; on the other hand, a dimethicone emulsion of diameter 4–5 µm performed better than the 0.6 µm one, although larger in size.
Finally, although the cationic amodimethicone with more affinity to damaged anionic hair performed better than the 0.6 µm emulsion, as expected, it gave a strong oily feel to dry hair, which could deter the consumer from using it (data not shown). Obviously, carefully choosing the oil and designing the cationic polymer to act as a deposition aid can significantly help to reduce fading; approximately two points were gained after 10 shampoos, ∆Efading = 7.4 ± 0.35, with formulation G (see Table 1) based on 2% w/w dimethicone, vs. 9.5 ± 0.3 with formulation C, free of silicone. The improvement is perceivable even to the untrained human eye.
Conclusions
The present article illustrates how complex the topic of color protection is—and as a result, how cumbersome (and costly) the development of such an expertise can be for formulators and suppliers. Water itself is a significant, if not the most significant, contributor to fading, but its use is difficult to avoid during washing. Limiting the exposure of colored hair to water is thus the first factor consumers should worry about, which is generally what hairdressers advise, especially the first days after coloration.
However, several additional factors negatively impact the durability of an artificial color, especially those that increase the exposure, penetration or removal of water from the hair shaft. Treatments like bleach and perm, and their interactions, have dramatic effects on color maintenance, much larger than each taken separately. Further, while the effects of each, separately, are comparable, their accumulation is highly detrimental. Consumers and formulators should therefore consider that the whole history of one’s hair can completely dominate the lasting effects of artificial hair color. Bleaching and perming, and the order in which they are conducted, as well as too aggressive blow-drying are all habits that contribute to repeatedly deforming hair and making it more prone to absorbing water, which certainly will aggravate color fading.
Interestingly, several of these separate factors have truly motivated the creation of new products and new claims. The negative effects on color retention of exposure to UV or heat, for instance, as well as the alleged stripping effect of surfactants are only a few examples that, in the end, have been responsible for new launches. The color protection segment, second in growth within hair care,14 is complex indeed but rich in problems to solve and in innovative solutions to be found.
Acknowledgements: The author is indebted to E. Choquet, D. Méchineau, O. Gravouil, C. Rouault, M. de Wilde, F. Geffray and F. Deschaseaux, who participated in acquiring the data presented in this paper. Further, the author wishes to acknowledge Rachel Grabenhofer for her work in editing this piece.
References
Send e-mails to [email protected].
1. Methodology based on an offline analysis, proprietary of the Solvay group; available upon request to the author.
2. D Bendejacq et al, Structured surfactant systems for high performance shampoos, Cosm & Toil 125 (11) 22–29 (2010)
3. J Kiplinger, A study of the influence of surfactants on hair color fading, presented at SCC Annual Meeting, New York (Dec 2009)
4. VM Brown, RG Crounse and DC Abele, An unusual new hair shaft abnormality: Bubble hair, J Am Acad Dermatol 15 1113–1117 (1986)
5. AS Savitha, S Sacchidanand and TN Revathy, Bubble hair and other acquired hair shaft anomalies due to hot ironing on wet hair, Int J Trichology 3(2) 118–120 (2011)
6. R Schueller and P Romanowski, ch 11 in Hair Care: From Physiology to Formulation, Allured Business Media, Carol Stream, IL, USA (2008) pp 73–75
7. C Zviak and J Milléquant, ch 7 in The Science of Hair Care, Informa Healthcare USA, Inc, London (2008) pp 232–233
8. LJ Wolfram, K Hall and I Hui, The mechanism of hair bleaching, J Soc Cosmet Chem, 21 875–900 (1970)
9. Contact angle data as a function of bleaching strength and duration (not shown); available upon request to the author.
10. C Zviak and A Sabbagh, ch 6 in The Science of Hair Care, Informa Healthcare USA, Inc, London (2008) pp 201–227
11. C Robbins, Chemical aspects of human hair bleaching, Soc Cosmet Chem 22 339–348 (1971)
12. AN Syed and H Ayoub, Correlating porosity and tensile strength of chemically modified hair, Cosm & Toil 117 (11) 57–64 (2002)
13. Pat WO12130997, Use of modified galactomannans for protecting dyed hair color from fading, Rhodia Opérations (Nov 7, 2012)
14. B Locke and J Jachowicz, Fading of artificial hair color and its prevention by photofilters, J Cosm Sci 56 407–425 (2005)
15. L Rebenfeld, HD Weigmann and C Dansizer, Temperature dependence of the mechanical properties of human hair in relation to structure, J Soc Cos Chem 17 525–538 (1966)
16. RY Lochhead, ch 41 in Hair Care: From Physiology to Formulation, Allured Business Media, Carol Stream, IL, USA 407 (2008)
17. S Marchioretto, The use of silicones as a color lock aid in rinse-off hair conditioners, J Cosm Sci 130–131 (2003)