Editor's note: This paper was first presented in short as a poster at the IFSCC Conference in Zurich.
The lipid matrix of the stratum corneum (SC), consisting primarily of ceramides, cholesterol and fatty acids, is crucial for the integrity of the skin barrier. Linoleic acid is an essential fatty acid, whose deficiency can lead to abnormal epidermal permeability barrier function.1, 2 In the case of its deficiency, linoleic acid is replaced with oleic acid, disturbing the normal formation of lipid lamellae in the SC.3
It has been proposed that topical treatment of linoleic acid could repair defective barrier function in detergent-treated skin.1 In addition, it has been shown that topical application of fatty acids in general could alter the intercellular lipid domains of the SC.4 A range of commercial and newly developed topical products containing linoleic acid was therefore used in the present study to assess, via transepidermal water loss (TEWL), the proposed effects:
- The protection potential, whereby the products are applied for a period of time before the insult to the skin barrier is carried out; in this case with sodium lauryl sulfate (SLS) solution; and
- The repair potential, whereby an insult with SLS to the skin barrier was followed by a period of product application.
TEWL: TEWL water loss is the key bioengineering parameter to assess skin barrier impairment and recovery.5 It is known to closely reflect the changes in the skin barrier function, hence it was chosen as the main skin variable to test in this study. A variety of protocols to assess TEWL exist in the literature, most of them based on published guidance documents.6 These protocols include a strict control of environmental conditions, specifically the temperature and relative humidity, as well as the air flow during measurements.
The instruments used to measure TEWL can be broadly divided in two groups based on open and closed chamber principles. For the present work, a typical representative open chamber instrumenta was used and compared with a relatively new generation of closed chamber instrumentsb. Special emphasis was placed on their sensitivities to detect small differences in TEWL. A complementary skin hydration testc also was carried out.
Materials and Methods
Materials: The anionic detergent SLS (≥ 99.0% purity), diluted in distilled water, was used to cause SC barrier impairment.7 A range of commercially available and newly formulated products containing linoleic acid was used to treat relevant test sites and 18 mm Finn chambers and 18 mm filter paper discs were used as occlusive patches.
Methods: Both studies were carried out per Ethics committee approval, following the guidelines of good laboratory practices. Before the start of the study, each participant signed an informed consent form.
Open chamber method: As noted, a typical open chamber instrumenta was used. The measuring head of the probe is a hollow cylinder with a diameter of 10 mm and a height of 20 mm. This contains two sets of sensors for detecting relative humidity and temperature, at different heights. The data obtained is converted into the flux density of the evaporating water, expressed in g/m2 hr.
The measurement time in this study was set to 20 sec. Using proprietary software, the mean TEWL value from the point of stabilization up to 20 sec was recorded in each case. Before the start of the study, the instrument was calibrated following manufacturer’s instructions. The reliability and validity of this method, under controlled experimental conditions, have been widely assessed.8
Closed chamber method: For the closed chamber methodb, the measurement chamber is a cylinder with a diameter of 8 mm and a length of 12 mm. The upper end of the probe is closed with a condenser that is maintained at a temperature of -7.65°C, below the freezing temperature of water.
When the probe aperture is placed against the skin, the water vapor emitted from the skin surface into the closed chamber is removed by its conversion into ice. An electronic cooler located in the condenser creates a controlled microclimate within the chamber, including constant relative humidity. Sensors for detecting relative humidity and temperature are mounted within the wall of the chamber, similarly to the open chamber system. However, the design of the probe enables TEWL measurements to be made independent of the external temperature and air movement.
The closed chamber measurement was completed after achieving a steady TEWL reading. Before the start of the study, the instrument was calibrated following manufacturer’s instructions. The reliability and validity of this method have previously been discussed.9
Protection Test Protocol
Fourteen healthy female volunteers having a mean age of 25.6, ranging from 19 to 42 years, participated in this study. No adverse reactions were recorded. The test area was the volar forearm. Three test sites of 3 x 3 cm, separated by 2.5 cm, were allocated symmetrically on both inner forearms of each participant. Regions near the wrist and flexural areas of the volar forearm were avoided. The study period spanned 14 days.
Three non-invasive measurements were taken in the following order: open chamber, closed chamber, then hydration test. All measurements were carried out in accordance to the relevant published guidelines. Baseline measurements were obtained after a 30-min acclimation at 21°C and 50% relative humidity.
Next, a 14-day supply of test products labelled as A, B, D, E and F were handed out to each participant, along with application instructions and a customized template showing the allocation of the products. This allocation among participants was balanced using the Latin Square Design, where test site C was the untreated control. Participants were instructed to apply test products twice daily by lightly massaging the site until complete absorption of the product. They returned for additional measurements after 7 and 14 days.
After collecting the TEWL and corneometer data on day 14, all test sites were subjected to SLS treatment under occlusion. A 200-µL aliquot of freshly made 5% w/v SLS was transferred on the round piece of filter paper, fitting into the 18 mm-Finn chamber. One Finn chamber was applied to each of six test sites, where it remained for 30 min. After removal of the SLS patches, the test areas were rinsed using running water and gently blotted with a tissue. The sites remained uncovered and exposed to air for 1 hr prior to the final set of measurements.
Repair Test Protocol
Thirteen healthy female volunteers having a mean age 25.4, ranging from 20 to 42 years, participated in this study. The test areas were both dorsal forearms, on which four test sites of 3 x 3 cm, separated by 2.5 cm, were allocated symmetrically. The study duration was 16 days.
After obtaining baseline measurements, researchers fitted all sites with 18-mm Finn chambers. Seven of them contained 200 µL of 1.25%w/v SLS, while test site C was covered by a filter disc soaked with 200 µL of distilled water. The occlusion lasted 24 hr, during which time participants were advised to refrain from vigorous activities and to shield the test sites against wetting. They were instructed to remove the SLS patches from the skin at a designated time and to expose the skin to air for at least 1 hr after the patch was removed.
Skin measurements were carried out 24 hr after patch removal, as suggested by Friebe et al.7 Following measurements after SLS damage, all sites were treated with test products except two: site C as a positive control (undamaged) and site H as a negative control (damaged with SLS). Participants were given a two-week supply and customized templates, as in study 1, and asked to use test products twice daily for two weeks. Final measurements were completed on day 16 of the study.
Statistical Analysis
Results were evaluated using analysis of variance (ANOVA) followed by Tukey HSD test for paired differences, with a 95% family-wise confidence level. A significance level of p < 0.05 was chosen. The data was plotted on graphsd as the mean ± SD; all statistical calculations were made using softwaree.
Results: Open Chamber, Study 1
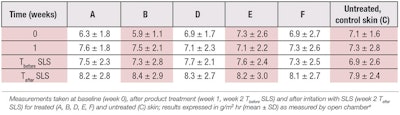
The TEWL values measured on inner forearms at different time points are shown in Table 1 and Figure 1, while Table 2 presents the results of the statistical analysis of the data. As expected, after exposure to 5% SLS for 30 min, a statistically significant increase in TEWL was observed at week 2 (Tafter SLS), compared with baseline values on all test sites (A-F) (p = 0.001). However, no statistically significant differences were found in TEWL between the different test sites (p = 0.99), indicating no differences among the test products.
Results: Closed Chamber, Study 1
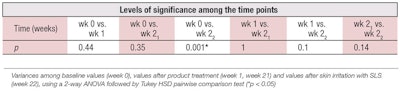
Table 3 and Figure 2 show the TEWL values obtained by closed chamber. While standard deviations were of the same order as with the open chamber, ANOVA analysis showed three sets of statistically significant data: TEWL values at baseline, week 1 and week 2 before the irritation with SLS, compared to week 2 after SLS (see Table 4).
Again, no statistically significant differences were found in TEWL between different test sites (p = 0.99; calculations not shown). These two sets of non-significant results, i.e., open and closed chamber studies, indicate not only no differences among the test products, but no differences between the test products and the untreated control. Therefore, study 1 TEWL measurements failed to show the protection potential of these test products.
Results: Skin Hydration, Study 1
Figure 3 illustrates the changes in hydration values of each test site at different time points. A statistically significant increase in skin hydration at week 1 compared to baseline values was detected. After irritation with SLS on week 2, skin hydration was drastically decreased, with statistically significant changes (p = 0.001) compared to baseline values, week 1 and week 2 before SLS irritation. However, no statistically significant differences were found in skin hydration among the test sites (p = 0.520).
Results: Open Chamber, Study 2
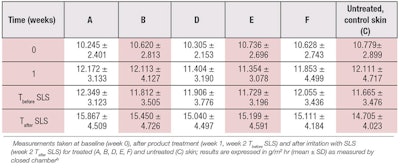
Figure 4 shows the TEWL values measured on dorsal forearms at different time points. After exposure to 1.25% SLS for 24 hr and subsequent stabilization for another 24 hr, a highly statistically significant increase was found in TEWL on day 2 compared to baseline values, as expected. No statistically significant differences were found in TEWL on day 16 compared with baseline values (p = 0.360), which indicated a successful barrier recovery.
The negative control site H, which was not treated with product after the SLS damage, performed similarly to the treated sites, demonstrating the power of skin’s natural barrier recovery. No statistically significant differences were found on site C (treated with the water patch) throughout the study, although an increase in TEWL was found on day 2.
Results: Closed Chamber, Study 2
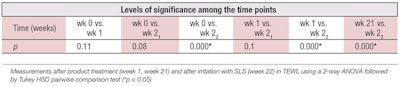
Figure 5 shows the mean TEWL values measured on the dorsal forearms at different time points using the condenser-chamber measuring method. It yielded the same statistical conclusions as the open chamber method between day 2 and baseline values (p < 0.05), and between day 2 and day 16 in all SLS-damaged test sites (p < 0.05). No statistically significant differences were found in TEWL on day 16 compared with the baseline values (p = 0.23).
The same conclusions as the open chamber measurements were drawn from the data obtained on control site C throughout the study. The results obtained in study 2 indicate the two methods used possess similar sensitivity when measuring relatively large differences in TEWL.
Results: Skin Hydration, Study 2
Figure 6 shows SC hydration values measured on the dorsal forearms at different time points. After exposure to SLS, a highly significant increase was found in skin hydration on day 2 compared with baseline values in all SLS-damaged test sites (A, B, D, E, F, G and H; p = 0.000). Also, a highly statistically significant decrease was found in skin hydration on day 16, after 2 weeks of product treatment, compared to day 2, as well as on day 16, compared with the baseline values (p < 0.05).
No significant difference was found on control site C, treated with water patches throughout the study. Also, no statistically significant differences were found in skin hydration between different test sites at different time points on day 16 (p = 0.85), indicating that natural recovery mechanism was as efficient as the applied treatments.
Discussion
It is widely accepted that TEWL measurements reflect the integrity of the skin barrier function and correlate well with its impairment.10, 11 In physical terms, TEWL is the flux of condensed water diffusing through the skin, from inside the body to the surface.9 The difficulty in getting correct measurements lies in the fact that all TEWL methods measure the water vapour flux in the air above the skin.
The measured flux depends on two key factors: 1) the rate of supply of water to the skin surface (biological factor) and 2) the rate of evaporation of water from the skin surface (microclimate factor). It is essential for the biological factor, not the microclimate factor, to determine the measured TEWL—a requirement achieved in different ways in the case of the open chamber approach, in comparison with the closed chamber one.
Instrument considerations: Being an open cylinder, the probe of the open chamber instrument possesses “natural ventilation,” which enables it to measure the condensed water flux with good accuracy.12 However, its design makes it sensitive to ambient air movements, which is a known difficulty with the open chamber method. In the present study, great care was taken to avoid any air movement during the measurement, but no special shielding of the probe was made. In addition, care was taken to keep the temperature of the probe constant by holding the probe in contact with the skin for 20 sec before the value was taken.
In contrast, the closed chamber eliminates the effects of air movement. For such a system to function accurately, however, the increasing amount of water vapor must be removed from the microenvironment. In the condenser-chamber method used here, this is achieved by trapping water molecules as ice on an electronically cooled condenser opposite the measurement orifice.13
The condenser provides the added benefit of maintaining constant and defined humidity at the skin surface, independent on the external environment. In addition, the instrument uses a precise gravimetric calibration,14 which is altogether an advantageous TEWL method. Indeed, when compared in terms of intrinsic measurement sensitivity with another type of open chamber instrumentf, the closed chamber approach was found to be ~40% more sensitive.13
This work aimed to compare the sensitivity of the two methods under the conditions of an in vivo volunteer study, and to draw practical conclusions for further use. It was found that the standard deviations (SDs) obtained by the two instruments were of the same order (see Table 1 and Table 3), leading to the conclusion that their large sizes were not due to the measuring method, but to the differences in skin physiology among different participants and different skin sites.
Given the large SD values obtained, it was not surprising that most ANOVA calculations did not produce significant differences, except when the skin was SLS-damaged (see Table 2 and Table 4). However, the condenser-chamber instrument produced two additional sets of significantly different TEWL results in study 1, showing that all TEWL measurements before the SLS damage were different from those obtained after 30 min of contact with SLS. No such differences between methods were found in study 2, where the SLS damage was higher and the rate of recovery more pronounced (see Figure 4 and Figure 5).
Test product considerations: Interesting results were obtained in both studies in terms of the efficacy of the test products. Not only were no significant differences revealed among the test products by either of the TEWL methods, but no differences were found between the products and the untreated control sites (see Figure 1 and Figure 2).
It is known that when the barrier function is disrupted, the activated homeostatic process increases lipid synthesis in the lamellar bodies in order to accelerate SC repair.15 In general, topical application of suitable products can enhance this process. However, the application of some lipid mixtures after barrier disruption have been found to delay this recovery.16 Furthermore, the correct molar ratio of the lipid mixture is recommended for optimal results,15 so it is possible the test products did not achieve the required optimal molar ratio. Hence, the barrier recovery was not enhanced in relation to the skin’s natural processes.
Skin hydration: Regarding the observed skin hydration changes, an expected decrease after 30 min of skin exposure to SLS occurred in study 1 (see Figure 3). It is known that a damaged skin barrier leads to an increase in TEWL, which results in a lower water content in the SC.17 However, in study 2, the large increase in skin hydration found 24 hr after the SLS patch removal (see Figure 5) was somewhat surprising.
It is known that increased hydration levels in skin are due to an occlusive effect of the Finn chamber18 but literature indicates this effect lasts approximately 20 min.19 It is therefore likely this hydrating effect found in study 2 was due to the swelling of corneocytes caused by prolonged contact with SLS and an increased water supply via TEWL.20, 21 The subsequent decrease in skin hydration after two weeks of topical treatment is thus proposed to be an exsiccation effect, caused by damage to the corneocytes.20
Conclusion
In the present work, the open chamber results of study 1 showed a significant increase in TEWL between the baseline and after SLS application in week 2, as expected, but no significance was detected between weeks or among test samples. The closed chamber results revealed two additional sets of significantly different data, showing a higher sensitivity.
In the second study, where barrier impairment was higher due to a 24-hour occlusive treatment, both methods resulted in the same statistical conclusions. Interestingly, no statistically significant difference was found two weeks after barrier damage between untreated sites and those treated with the test samples, indicating the natural barrier recovery of damaged healthy skin was efficient.
The results of this study indicate the closed chamber method possesses a higher sensitivity than the open chamber method when dealing with smaller differences in TEWL. It produced a higher number of statistically significant findings under the same experimental conditions. However, when larger variations in TEWL were detected between test sites, the findings obtained by the open chamber method were consistent with the closed chamber findings.
References
- PM Elias, BE Brown and VA Ziboh, The permeability barrier in essential fatty acid deficiency: Evidence for a direct role for linoleic acid in barrier function, J Inves Derm 74 230-3 (1980)
- KR Feingold and PM, The environmental interface: Regulation of permeability barrier homeostasis, in M Loden and HI Maibach, eds, Dry Skin and moisturizers: Chemistry and Function, Boca Raton, FL, CRC Press (2000)
- KR Feingold, The role of epidermal lipids in cutaneous permeability barrier homeostatis, J Lipid Res 48 2531-2546 (2007)
- BJ Aungst, The influence of fatty acids and fatty alcohols on skin permeability, in M Loden and HI Maibach, eds, Dry Skin and Moisturizers: Chemistry and Function, Boca Raton, FL, CRC Press (2000)
- KA Grice, Transepidermal water loss, in A Jarrett, ed, The Physiology and Pathophysiology of the Skin, Academic Press, London (1980) pp 2116–2146
- V Rogiers and the EEMCO Group, EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences, Skin Pharmacol Appl Skin Physiol 14 117–128 (2001)
- K Friebe, I Effendy, and H Loffler, Effects of skin occlusion in patch testing with sodium lauryl sulfate, Brit J Dermatol 148, 65-69 (2003)
- C Rosado, P Pinto and LM Rodrigues, Comparative assessment of the performance of two generations of Tewameter: TM210 and TM300, Intl J Cosm Sci 27 237-241 (2005)
- RE Imhof, MEP De Jesus, P Xiao, LI Ciortea and EP Berg, Closed-chamber transepidermal water loss measurement: Microclimate, calibration and performance, Intl J Cosm Sci 31 97-118 (2009)
- M Willoughby and HI Maibach, Cutaneous biometrics and claims support, in LB Austed, Cosmetic Claims Substantiation, New York, Marcel Dekker (1998)
- KP Wilhelm, G Freitag and HH Wolff, Surfactant-induced skin irritation and skin repair. Evaluation of the acute human irritation model by noninvasive techniques, J Am Acad Dermatol 30 944-999 (1994)
- J Nuutinen, Measurement of transepidermal water loss by closed-chamber systems, in J Serup, GBE Jemec and GL Grove, eds, Handbook of Non-invasive Methods and the Skin, 2nd edn, Boca Raton, CRC Taylor & Francis (2006)
- RE Imhof, EP Berg, RP Chilcott, LI Ciortea, FC Pascut and P Xiao, New instrument for measuring water vapour flux density from arbitrary surfaces, IFSCC Magazine 5(4) 297-301 (2002)
- P Xiao, W Wong, AM Cottenden and RE Imhof, In vivo stratum corneum over-hydration and water diffusion coefficient measurements using opto-thermal radiometry and TEWL instruments, Int J Cos Sci 34(4) 328-331 (2012)
- M Denda, Role of lipids in skin barrier function, in T Forster, ed, Cosmetic Lipids and the Skin Barrier, New York, Marcel Dekker (2002)
- MQ Man, KR Feingold and PM Elias, Exogenous lipids influence permeability barrier recovery in acetone-treated murine skin, Arch Dermatol 129 728-738 (1993)
- K De Paepe, D Roseeuw and V Rogiers, Repair of acetone and sodium lauryl sulfate-damaged human skin barrier function using topically applied emulsions containing barrier lipids, JEADV 16 587-94 (2002)
- H Zhai and HI Maibach, Occlusion and barrier function, in H Zhai, KP Wilhelm, HI Maibach, eds, Marzulli and Maibach’s Dermatotoxicology, 7th edn, Boca Raton, CRC Taylor & Francis (2008)
- T Agner and J Serup, Time course of occlusive effects on skin evaluated by measurement of transepidermal water loss (TEWL): Including patch tests with sodium lauryl sulfate and water, Cont Derm 28 6-9 (1993)
- M Gloor, B Senger, M Langenauer and J Fluhr, On the course of the irritant reaction after irritation with sodium lauryl sulfate, Skin Res Technol 10 144-148 (2004)
- R Darlenski and JW Fluhr, Moisturizers and emollients, in JW Fluhr, ed, Practical Aspects of Cosmetic Testing, Heidelberg, Springer (2011)