In 2010, news was reported of a British man who had never before had an allergic reaction, but upon use of one popular deodorant spray, almost died. According to the story, “after spraying himself with it, the man began to develop an itchy rash that quickly developed into anaphylactic shock, which left him unconscious.”1 He had used the same brand before without any problems, but apparently this was a new line that caused his severe reaction.
An unrelated story in 2013 reported that a male student with a life-threatening allergy to another deodorant spray was hospitalized for two weeks, for the second time, which led high school administrators to ask students to refrain from using the product.2 The student experienced rash-like symptoms, difficulty in breathing and swelling of the throat, and the school nurse used an epinephrine injection to stop him from going into anaphylactic shock.
Another case, published in 2009,3 describes a woman who experienced anaphylactic shock from use of a facial cream. Initially, she was seeking medical attention for a severe yet localized skin reaction to it. Upon arrival at the clinic, her face was extremely edematous, so much so that she could barely open her eyes. The edema was strictly limited to facial regions where the cream had been applied the previous evening. According to the report, this reaction happened although she had been using the same cream regularly. Submitting the closed container of cream to the physician, the woman collapsed, presenting signs of anaphylactic shock. Immediate intervention including adrenaline administration proved sufficient. The cause of this transition from local to systemic reaction was unclear, but speculations included repeat exposure to traces of the product present on the cover of the closed container.
Scenarios such as these, while rare, raise not only industry and regulatory concerns, but also public concern over the safety of personal care products—regardless of the conditions under which the reactions occurred, or likelihood they could happen again. Individuals who are aware of their increased sensitivity clearly should be aware of and avoid contact with anything containing the allergen, since even trace amounts could trigger such reactions. In addition to ethics, with the reputation of multinational brands on the line, these concerns have given rise to a focus on pharmacovigilance in the cosmetics industry. And considering the potential for reactions between cosmetic ingredients and hypersensitive skin, the present article will focus on this skin type; first by outlining the mechanisms of type I and IV hypersensitization, then by focusing on type I immunogenic vs. nonimmunogenic urticaria. Also discussed are potential urticariogens in cosmetics, and suggestions for future direction.
Skin Immunity
Beyond a mere barrier, the skin is by far the largest immune response organ of the body and its immunity can be classified in two main categories: innate and acquired. Innate includes the fundamental protection with which individuals are born, such as the stratum corneum barrier, pH mantle, biota hosts and antimicrobial lipids and peptides that are either part of the barrier or secreted in sweat and sebum. Acquired immunity includes protection mechanisms attained throughout one’s lifespan as a result of exposure to threats. These include antibodies generated from exposure to allergens and different immune memory cells, as well as the expression of genes and proteins that are activated upon insult. Acquired immunity is also dependent upon genetic predisposition and exposures due to lifestyle.
The complicated immunity presented by skin is one that strives for fast and efficient correction of the tissue and eradication of the threat. However, this correction process, if strong, persistent or connected with genetically predisposed components, may come with the price of scarring, wrinkles and other imperfections. Acknowledging the skin is a protection organ, why would it be surprising for skin to react upon the application of chemicals?
Most skin care companies follow industry regulations and guidelines, and conduct recommended safety assessments to ensure no adverse effects occur after a product is launched. However, such testing incurs challenges of person-to-person variably, and the inherent difficulty in testing a large number of subjects and projecting their responses to billions of consumers. These complications, in addition to the complexity of reactions themselves and limitations in their full scientific understanding, point toward gaps in the current suggested test protocols, allowing adverse effects to occur.
For example, one major gap relates to type I hypersensitization, or contact urticaria reaction. This is an immediate reaction that may lead to systemic complications such as the shock reaction previously described, and unlike primary irritation, is not confined to the upper layer of the skin, but penetrates to the lower dermis. Testing for contact urticaria is not part of published industry guidelines, although it has been reportedly induced by some commonly used skin care ingredients and can lead to a deadly anaphylaxis response.4
Types of Hypersensitization
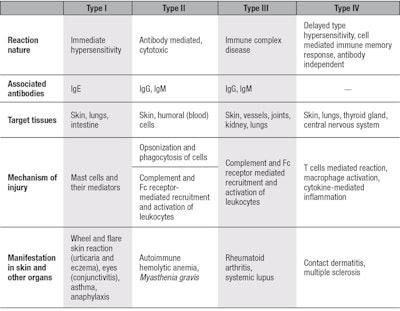
Hypersensitivity is a state of increased reactivity or sensitivity to a stimulus that is recognized as a threat. Hypersensitization reactions are divided into four categories, referred to as types I-IV, with type I being the most severe. These differ by target organ, clinical manifestation and the biomarkers involved (see Table 1). Being an immune response organ, the skin may present all four reactions. However, types II and III are more commonly expressed in other organs,5 therefore types IV and I in relation to skin are detailed here.
Type IV: The most studied among skin sensitization reactions is allergic contact dermatitis. The prevalence of contact allergy in the total European population is estimated at 20% and is rising worldwide. This type IV reaction involves a delayed skin rash with blistering and oozing of the skin. It usually is attributed to the use of accelerators, such as skin penetration enhancers, long-term occlusion and chronic exposure to the allergen.6
The most commonly reported allergens in topically applied products are nickel as well as fragrances and preservatives; about one-third of all allergies to cosmetic products are due to fragrance.7 The highest risk factors for all types of allergies include acquired inflammatory skin disorders as well as inherent genetic variances that result in higher susceptibility. Since the induction and severity of sensitization reaction is directly correlated to the level of exposure, a breach in skin barrier is a major contributing factor.
The development of a type IV hypersensitization reaction in skin can generally be described as a four-step process:
- Allergen bioavailability, i.e., penetration via the stratum corneum in sufficient concentration;
- Stimulation of local trauma, leading to inflammation and cytokine expression;
- Formation of the sensitizing conjugate hapten protein; and
- Activation of T lymphocytes, after memory cells have been acquired.
In the currently established model for delayed sensitization, dendritic Langerhans cells react to potential allergens by interacting with epidermal keratinocytes, and then migrate to the local lymph nodes to activate naive T cells.8 After T cells acquire memory, and when a subsequent exposure to the allergen occurs, T cells are activated, proliferate and act at the dermal-epidermal junction to deliver inflammatory toxic mediators, and induce inflammation and accelerated keratinocyte deaths.
Type I: The most serious and rare form of hypersensitization, type I is an immediate and potentially life-threatening reaction. Type IV sensitization is a T-cell and macrophage-mediated reaction that must be systemic to involve the circulation and lymphatic system, whereas type I is a mast cells-mediated reaction that when immunogenic, involves the generation of Immunoglobulin E (IgE) antibodies. Mast cells that are activated in type I are dermis-residing cells that contain granules storing a variety of mediators. When activated, a mast cell rapidly releases its granules, discharging various hormonal, lipid and peptide-derived mediators into the interstitium. Mast cells can be stimulated to de-granulate by: direct injury, e.g., via physical or chemical means such as opioids, alcohols and certain antibiotics; the cross-linking of IgE receptors; or by so-called immune related complement proteins.9
In allergic reactions, mast cells remain inactive until, when immunogenic, an allergen binds to the IgE antibody that is already associated with the cell. This means a prior exposure to the antigen produced antibodies by the immune system confined to the mast cell, readying the cell for exposure and activation. Other membrane activation events can prime mast cells for subsequent degranulation. These include mechanical pressure and exposure to drugs/compounds. Since the skin mast cells reside in the lower vasculated dermis, in order for type I sensitization to occur by an external trigger, the insulting chemical/reaction must penetrate through the stratum corneum and migrate through the living epidermis; for example, consider a bee sting.
In relation to skin, type I allergy is named urticaria, but factors involved in atopic dermatitis can also occur. In fact, the puzzling aspect is that type IV, i.e., cell-mediated, local allergic reactions do not necessarily exclude type I, immunoglobulin-mediated reactions including anaphylaxis. The two types of reactions, even only to contact allergens, might occur in the same person successively or simultaneously. Symptoms typically include hives, itch and pruritus, flushing or swelling of the lips. Some individuals also report a burning sensation of the skin rather than itch. Swelling of the tongue or throat occurs in up to about 20% of cases; worldwide, 0.05–2.0% of the population is estimated to have experienced anaphylaxis at some point in their life, and rates appear to be increasing.10
Unlike allergic contact dermatitis—which has a delayed nature and upon re-exposure to the allergen, reacts over 1–3 days—urticaria occurs within minutes after the allergen or mast cell activator comes into contact with the target organ. It is therefore considered immediate hypersensitivity. Activation of mast cells leads to the release of mediators such as histamines, prostaglandins, leukotrienes and heparin that enhance vascular permeability to produce edema.
Although in most cases urticaria is triggered upon dermal exposure, in some cases the respiratory or gastrointestinal systems may be the route of entry for an immunogenic urticariogen. This reaction can therefore appear on non-atopic, normal skin upon first exposure and may be due to the direct activation of dermal mast cells that release histamine and other pro-inflammatory mediators. Therefore, it is nearly impossible to predict non-immunogenic urticaria. Mast cells are the key effector types in IgE mediated immediate hypersensitivity and allergic disorders, as well as in protective immune responses to certain parasites and bacteria.11
Contact Urticaria to Cosmetic Compounds
Immunogenic urticaria reactions to a variety of compounds are reported in the literature. Most studies are conducted on ingredients contained in processed foods such as preservatives and colorants,12 or on specific food groups such as dairy, nuts and meats. In addition, immunogenic urticaria can be triggered by drugs including antibiotics such as ampicillin and bacitracin, or metals such as nickel and copper.
Cosmetic ingredients and formulations also potentially contain urticariogens, either as ingredients purposely included, or as contaminants.4 Example urticariogens are oils from nuts such as peanuts, sesame or sunflower; fruit and vegetable extracts including cucumber or soybean; fragrance and flavor, e.g., vanilla or menthol; and plant extracts from algae, lily or lime. Interestingly, even chamomile extract, known to exhibit anti-inflammatory properties, can trigger immunogenic urticaria.4 Other potential urticariogens are: preservatives such as benzoic acid, benzyl alcohol and parabens; emulsifiers, including polysorbates; the solvents propylene glycol, ethanol and others; contaminant enzyme residues from fermentation/biological production; metal impurities; remaining monomers from polymer reactions; and disinfectants and detergents used in production that have not been thoroughly removed.
In contrast to immunogenic reactions, non-immunogenic urticaria remains localized and should not cause systemic symptoms. It is estimated that most cases of contact urticaria are not reported and go untreated unless they progress to a severe condition.13, 14 Individuals affected typically consider these reactions part of their “sensitive skin” condition. As a result, appropriate tests to identify the condition are not performed and the abundance of the phenomenon in the general population remains unknown. Example ingredients potentially included in cosmetic formulations that can trigger non-immunogenic contact urticarial are: benzoic acid, sorbic acid, cinnamic acid, cinnamic aldehyde, pine oil and propylene glycol.4 Concentrations of these components even at as low as 0.1–0.2% can trigger reactions.
It should be noted that the list of triggers for non-immunogenic reaction is smaller than for immunogenic reaction, and that there are compounds such as benzoic acid and propylene glycol that can cause both types of reactions. The challenge in pre-identifying non-immunogenic urticarigens lies in the fact that the mechanism for such reaction has not been established and screening models are absent. While urticaria can be restricted to the skin, the mere activation of mast cells is of concern because these mediators, when released chronically, can develop into a systemic or skin-confined chronic condition.
Summary and Future Direction
Exposure to potential allergens or skin reactants does not always lead to a response and when the threshold for clinical manifestation is not met, the exposed individual may not recognize the risk. Thus, exposure to threat will be maintained. It is clear that in many cases, especially involving the progression of insult to chronic skin inflammatory diseases such as psoriasis and atopic dermatitis, genetic pre-disposition is a key factor. However, in some cases, while individuals may be exposed to the same invader at the same concentration, only few will express a reaction. The difference may simply be associated with the integrity of the skin barrier—which is easily breached in reacting individuals while highly protective in others.
Barrier properties can dramatically differ between individuals and is usually assessed by measuring trans-epidermal water loss (TEWL). Moreover, the same individual can be reactive to exposure at one time point and non-reactive in another. This is indicative that barrier properties can be modified by environment, lifestyle or age. It is therefore almost impossible to predict when an individual will be reactive to exposure.
In the past two decades, an increasing number of individuals in the Western world are reporting to a physician’s clinic with complaints of “sensitive skin.”15 In some cases, this syndrome leads to their refraining from the use of any topically applied products for esthetic reasons. It is not clear if this increase in reporting is a result of higher awareness or lies in true biochemical changes, but it is now well-recognized in the medical community, and a few studies point to underlying mechanisms and differences in biomarkers between “normal” and “sensitive” skin.
It is common practice in the cosmetic industry to run the repetitive insult patch test (RIPT) for the prediction of potential primary irritation or skin sensitization. However, the type of sensitization detected in RIPT is type IV delayed sensitization and not type I. The RIPT procedure involves the application of a patched component or formulation that is removed for evaluation of the potential reaction 24 hr after application. Therefore, if an immediate urticaria reaction did occur, it would have disappeared by the time of scoring.
This practice also does not protect from developing type I sensitization in susceptible individuals, anaphylaxis included. Clearly, filling gaps in testing protocols for the screening of skin for adverse reactions should be a work in progress. Reports of reactions to topically applied products should be shared, reviewed and investigated within the scientific community in order to build a database, and to better understand the prevalence and incidence of reactions in the population.
References
- www.dailymail.co.uk/news/article-1239864/The-ad-promises-lust-Lynx-deodorant-kills-man-suffers-allergic-reaction.html (Accessed Jan 6, 2014)
- http://articles.mcall.com/2013-03-20/news/mc-bethlehem-school-axe-body-spray-20130320_1_body-spray-allergic-reactions-students-spray (Accessed Jan 8, 2014)
- www.researchgate.net/researcher/32995998_Vesna_Kos/ (Accessed Jan 8, 2014)
- S Amin, HI Maibach and A Lahti, Contact urticaria syndrome, in Dermatology: Clinical and Basic Science, CRC Press, NY (1997)
- H Loffler, J Aramaki, I Effendy and HI Maibach, Sensitive skin, in Dermatotoxicology, H Zhai and HI Maibach, eds, New York, CRC Press (2004) pp 123-135
- M Peiser et al, Allergic contact dermatitis: Epidemiology, molecular mechanisms, in vitro methods and regulatory aspects, Cell Mol Life Sci 69 763-781 (2012)
- DI Orton and JD Wilkinson, Cosmetic allergy: Incidence, diagnosis and management, Am J Clin Dermatol 5(5) 327-37 (2004)
- A Richter et al, Human T cell priming assay (hTCPA) for the identification of contact allergens based on naive T cells and DC—IFN-γ and TNF-α readout. Toxicol In Vitro 27 1180-1185 (2013)
- C Prussin and DD Metcalfe, IgE, mast cells, basophils and eosinophils, J Allergy Clin Immunol 111 (2 suppl) S486–94 (2003)
- FE Simons, World Allergy Organization survey on global availability of essentials for the assessment and management of anaphylaxis by allergy-immunology specialists in health care settings, Annals of Allergy, Asthma & Immunology 104 (5) 405–12 (May 2010)
- T Kawakami, T Ando, M Kimura and BS Wilson, Mast cells in atopic dermatitis, Curr Opin Immunol 21(6) 666-678 (2009)
- A Goossens, Contact allergic reaction to cosmetics, J of Allergy 6 467071 (2011)
- E Varjonen. L Petman and S Makinen-Kiljunen, Immediate contact allergy from hydrolyzed wheat in cosmetic cream, Allergy 55 294-296 (2000)
- S De Paz Arranz, AP Montero, LZ Remon and MIM Molero, Allergic contact urticaria to oatmeal, Allergy 57 1215 (2002)
- J Escalas-Taberner, E Gonzalez-Guerra and A Guerra-Tapia, Sensitive skin: A complex syndrome, Acta Dermosifiliogr 102(8) 563-71 (Oct 2011)
Additional Reading
- J Coverly, L Peters, E Whittle and DA Basketter, Susceptibility to skin stinging, nonimmunologic contact urticaria and acute skin irritation; Is there a relationship? Contact Dermatitis 38 (1998) 90-95
- C Di Giovanni, V Arcoraci, L Gamvardella and L Sautebin, Cosmetovigilance survey: Are cosmetics considered safe by consumers? Pharmacological Research 53 (2006) 16-21
- M Marriott, J Holmes, L Peters, K Cooper, M Rowson and DA Baketter, The complex problem of sensitive skin, Contact Dermatitis 53 (2005) 93-99