Skin is a complex biological model that is nonetheless highly approachable. Several methods exist to study it, including animal skin and human skin, both in vitro and in vivo models; regional variation models; and stem cell and hair follicle biological models. Through such models, pharmaceutical preparations in dermatology have been found to affect cell regulation. However, the US Food and Drug Administration (FDA) usually exempts dose justifications for dermatologic preparations. With oral and parenteral dosing, dose justification is generally done during phase II. With topicals, a relatively arbitrary percentage is often dosed—without the benefit of dose justifications. As a result, the presence of any hormetic effects could be missed.
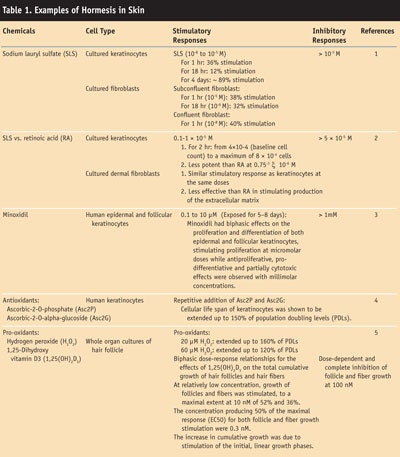
Dermatology literature indicates that several cell types in the skin provide evidence of hormetic-like biphasic dose/concentration-response relationships. A brief listing of these cell types, relationships and the quantitative features of dose responses is presented in Table 1. This review examines hormetic effects of various agents on skin biology. Recognition of this emerging biological phenomenon in dermatology could lead to markedly improved integrative assessments of animal/human skin responses to toxic substances and pharmacological agents, as well as endogenous agonists.
Mechanisms of Hormesis
In toxicology, hormesis is a dose-response phenomenon characterized by a low-dose stimulation effect, followed by a high-dose inhibition effect. One cosmetic example of this is demonstrated by the effect of the surfactant sodium lauryl sulfate on keratinocytes in vitro: dose response studies demonstrate that low levels increase or stimulate cell replication, while higher levels decrease or inhibit it. 1 This biphasic dose response has been noted in a wide range of biological model systems ranging from immunology to cancer biology.6, 7
Calabrese6, 7 has been the primary advocate in bringing this interesting and not uncommon phenomenon to the attention of the scientific community. As noted by Calabrese, the quantitative features of the hermetic-like biphasic dose response are remarkably similar with respect to the amplitude of the stimulatory response, the width of the stimulation, and the relationship of the maximum stimulatory response to the zero equivalent point (ZEP), i.e., the threshold. Typically, the low-dose hormetic biphasic dose response stimulation is modest, with a maximum stimulation from 30% to 60% greater than controls, and produces a similar appearance in different cell types with various chemicals.7
Most stimulatory ranges were less than 100-fold, having averages of 10- to 20-fold, measuring back from the ZEP. These low-dose stimulatory responses often occur following an initial disruption in homeostatis and appear to represent a modest overcompensation response.
It is believed that this modest stimulatory responsiveness is the result of a compensatory process that slightly “overshoots” its goal of the original physiological set-point, ensuring that the system returns to homeostasis without unnecessary and excessive overcompensation.8 Therefore, it is important to follow the dose-response relationships over time to better define their quantitative features.
While initial interest has been focused on the hormetic effects of pollutants and toxic substances on biological systems,9 interest has expanded to include pharmacological agents and phyto-compounds, as well as endogenous agonists.7 Hormetic-like biphasic dose response relationships appear to be highly generalizable; that is, such responses do not appear to be restricted by biological model, endpoint or chemical/physical stressors.7
Several investigations have attempted to assess mechanisms that could account for the hormetic-like biphasic dose-response relationship. In general, there is no single mechanism that can account for the plethora of hormetic relationships. Nonetheless, a common molecular tactic by which biphasic dose-response relationships are displayed involves the presence of two receptor subtypes affecting cell regulation, one with high and the other with low affinity for the agonist but with notably more capacity (i.e., more receptors).7 Such an arrangement may lead to the biphasic dose response, with the high-affinity receptor activated at low concentrations, which stimulates DNA synthesis and cellular proliferation; and the low-affinity/high-capacity receptor becoming dominant at higher concentrations, decreasing the cell proliferative response. This is a general pharmacological mechanism in that it is employed for a large number of receptor-based responses, from cancer cells to neutrophil chemotaxis and many others.
Hormesis Considerations in Toxicology Assessments
Calabrese and Blain10 have compiled a hormesis database containing 5,600 hormetic-like dose response relationships and more than 900 agents from a broadly diversified spectrum of chemical classes and physical agents. This compilation stresses the robustness of the published body of work supporting the hormetic dose-response hypothesis. However, despite the extensive observation of hormetic dose-response relationships, most studies have assessed cellular responses and few have continued the work with animal and human models, normal or diseased, or have assessed the simultaneous responses of different systems to the same agent. In vivo studies are necessary to provide an integrative assessment of the whole animal/human response to various agents, to document any discrepancies between in vitro and in vivo responses, and to clarify the clinical implication of hormesis.
Another issue to consider regarding hormesis, as proposed by van Der Woude,11 is the need to modify riskassessment paradigms to take hormesis into account. Further, Rietjens and Alink12 suggest that toxicology studies should examine not only the adverse effects at high levels of exposure, but also the complex biological effects, adverse and beneficial, at low levels of exposure. Low-dose toxicology and pharmacology will contribute to developing better methods of low-dose risk assessment of chemical compounds and their effect on carcinogenesis by considering that the ultimate biological effect of a chemical may vary with its dose, the endpoint or target organ considered, cellular interactions, and/or the combined exposure with other chemicals.
Conclusions
The skin is an excellent candidate to gain entrance into the biology of hormesis due to its accessibility; its complex nature, with highly differentiated cell types and various subsystems—i.e., keratinocytes, melanocytes, Langerhans’ cells, fibroblasts, epidermis, dermis, hair follicle, eccrine, apocrine and sebaceous units; and the availability of specialized noninvasive technologies for in vivo studies.13, 14 In addition, skin is among the first organs that has been analyzed using DNA microarrays for skin cancers, melanomas, basal cell carcinomas, squamous cell carcinomas, psoriasis and other inflammatory disorders, as well as for stem cell biology, the biology of epidermal keratinocytes, and so forth.15 DNA microarray studies could be an excellent tool to elucidate the mechanisms of hormesis in skin biology. In short, a better understanding of hormesis could lead to different strategies for risk assessment processes employed in the fields of cosmetic dermatology, toxicology and pharmacology.
The chemistry of many personal care products is based on formulations and formulators’ convenience; when dose response data for more individual ingredients becomes available, more effective personal care products can be formulated.
Reproduction of all or part of this article is strictly prohibited.
References
Send e-mail to [email protected]
1. E Bloom, M Sznitowska, J Polansky, ZD Ma and HI Maibach, Increased proliferation of skin cells by sublethal doses of sodium laurel sulfate, Dermatology 188 263–268 (1994)
2. J Varani, A Astrom, CEM Griffiths and JJ Voorheers, Induction of proliferation of growth-inhibited keratinocytes and fibroblasts in monolayer culture by sodium lauryl sulfate: Comparison with all-trans retinoic acid, J Invest Dermatol 97 917–921 (1991)
3. N Boyera, I Galey and BA Bernard, Biphasic effects of minoxidil on the proliferation and differentiation of normal human keratinocytes, Skin Pharmacol 10 206–220 (1997)
4. S Yokoo, K Furumoto, E Hiyama and N Miwa, Slow-down of age-dependent telomere shortening is executed in human skin keratinocytes by hormesis-like effects of trace hydrogen peroxide or by antioxidative effects of pro-vitamin C in common concurrently with reduction of intracellular oxidative stress, J Cell Biochem 93 588–497 (2004)
5. CS Harmon and TD Nevins, Biphasic effect of 1,25-Dihydroxyvitamin D3 on human hair follicle growth and hair fiber production in whole-organ cultures, J Invest Dermatol 103 318–322 (1994)
6. EJ Calabrese, Hormetic dose-response relationships in immunology: Occurrence, quantitative features of the dose-response, mechanistic foundations and clinical implications, Crit Rev Toxicol 35 (6) 89–295 (2005)
7. EJ Calabrese, Cancer Biology and Hormesis: Human tumor cell lines commonly display hormetic (biphasic) dose responses, Crit Rev Toxicol 35 (6) 463–582 (2005)
8. EJ Calabrese, Overcompensation stimulation: A mechanism for hormetic effects, Crit Rev Toxicol 31 (4, 5) 425–470 (2001)
9. EJ Calabrese and LA Baldwin, The dose determines the stimulation (and poison): Development of a chemical hormesis database, Int J Toxicol 16 545–559 (1997) 1
0. EJ Calabrese and R Blain, The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview, Toxicol Appl Pharmacol 202 289–301 (2005)
11. H van der Woude, GM Alink and IMCM Rietjens, The definition of hormesis and its implications for in vitro to in vivo extrapolation and risk assessment, Crit Rev Toxicol 35 603–607 (2005)
12. IMCM Rietjens and GM Alink, Future of Toxicology: Low-dose toxicology and risk-benefit analysis, Chem Res Toxicol 19(8) 977–981 (2006)
13. HI Maibach, Dermatologic Research Techniques, Boca Raton, FL: CRC Press (1996)
14. P Elsner, E Berardesca, KP Wilhelm and HI Maibach, Bioengineering of the Skin, Boca Raton, FL: CRC Press (2001)
15. M Blumenberg, DNA microarrays in dermatology and skin biology, J Integrat Biol 10(3) 243–257 (2006)