Sunscreens are designed to provide broad-spectrum protection to skin against the damaging effects of ultraviolet (UV) radiation. In doing so, they prevent sunburn and help to reduce the risk of skin cancer.1 Today’s consumers are increasingly concerned about the side effects of photoaging, e.g., fine lines, wrinkles, sagging, age spots, etc., which compromise skin’s youthful appearance.
Photoaging is believed to be triggered primarily by reactive oxygen species (ROS).2 ROS form within the epidermis and dermis when endogenous chromophores such as urocanic acid, nicotinamide adenine dinucleotide (NADH), melanin and collagen absorb UV radiation then dissipate the absorbed energy through pathways that sensitize the formation of ROS. Just a few of the ROS found in the skin include singlet oxygen (1O2), hydrogen peroxide (H2O2) and superoxide radical anions (O2-•). These ROS can react with lipid membranes, proteins, DNA and most other molecules in their paths. While intrinsic antioxidant mechanisms within the extracellular and intracellular spaces of the epidermis and dermis counteract some UV-induced ROS, these mechanisms can become overloaded easily, resulting in ROS-mediated cell damage.3, 4
To minimize ROS levels while concurrently preventing sunburn, sunscreens are being formulated with both UV filters and efficacious antioxidants. However, there is a lack of understanding regarding how well antioxidants reduce ROS levels for different degrees of UV attenuation. For example, do higher SPF formulations need the same level of antioxidants as lower SPF formulations to minimize ROS levels? In this report, two-photon fluorescence microscopy is used to evaluate the extra-protective benefits afforded by an antioxidant combination of 0.5% vitamin E and 0.9% diethylhexyl syringylidene malonate (DESM) in sunscreens of low to high SPF by assessing its ability to reduce UV-induced ROS levels within the stratum corneum (SC).
Materials and Methods
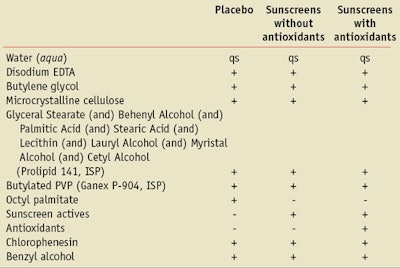
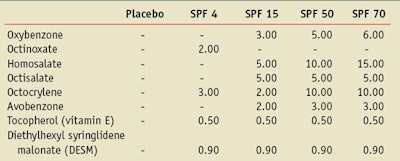
Placebo and sunscreen formulations of different SPF levels were obtained from Merck Consumer Care and labeled with codes to prevent bias during data analysis. Sunscreens having SPFs of 4, 15, 50 and 70 and formulated using various combinations of UV filters and combinations, with or without tocopherol or DESMa, were evaluated. A generalized comparison of the formula compositions appears in Table 1.
All formulas were prepared using the same types and levels of inactive excipients. The placebo formula substituted octyl palmitate for the sunscreen actives and removed the antioxidants. The specific types and levels of sunscreen actives and antioxidants contained in the formulas appear in Table 2. These formulations are representative of products available commercially. Additional supplies necessary to study the formulations included: an ROS fluorescence probe, dihydrorhodamineb, epidermal skin equivalentsc, indicator-free cell culture mediad and a phosphate-buffered saline (PBS) solutione.
Each epidermal tissue insert was incubated at 37°C and 5% CO2 in the cell culture medium for 24 hr to bring the tissues to metabolic equilibrium, after which the medium was refreshed. ROS were detected indirectly from the conversion of non-fluorescent dihydrorhodamine (DHR) to fluorescent rhodamine-123 (R123) following reaction with ROS.2–3 A 100-μL aliquot of 45 μM DHR (1:99, ethanol:PBS) was applied to the tissue surface and the tissues were incubated for 30 min at 37°C and 5% CO2. The DHR solution was then removed and the tissues were rinsed with 100-μL aliquots of PBS.
The cell culture media was replaced with 0.9 mL PBS. Using a positive pressure pipette, 2 mg/cm2 of test formulation was applied to tissue surfaces, rubbed into the skin with the tip of a clean, glass rod for 5 sec and placed in a dark drawer for 1 hr with the culture plate lid removed. Placement in the darkened drawer allowed the formulation films to set up under a less humid environment, compared with the incubator, and prevented the conversion of DHR to fluorescent R123 from ambient light exposure in the lab. After 1 hr, the tissues were removed from their inserts using forceps and set with the SC side facing up on a piece of filter paper wetted with PBS on a glass slide. Then they were irradiated with 1 minimal erythemal dose (MED) of UVB-UVA (20 mJ/cm2) solar simulated radiationf, covered with a coverslip and imaged using two-photon fluorescence microscopy (TPM).
TPM has been successfully used to monitor UV-induced ROS generation within the skin.5, 6 As described above, UV-induced ROS react with non-fluorescent DHR, leading to the oxidized product fluorescent R123.5, 7–10 The tissue is placed on the microscope, where R123 can then be excited by the simultaneous absorption of two near-IR photons. The fluorescence from R123 is detected as a function of x-y-z location. This imaging technique identifies the location of converted DHR and indirectly shows the formation of ROS in the tissue.
Two-photon excitation is the method of choice for imaging chemical phenomena in skin. It uses fast, pulsed near IR photons that, unlike one-photon UV and visible confocal microscopy, are inherently confocal. This affords sectioned imaging to depths of ~100 μm with < 1 μm spatial resolution and reduces the photobleaching of the fluorescence probe and negligible photodamage to the tissue sample. A custom-built scanning two-photon fluorescence microscope was used for this study.11
The skin treated with the placebo (i.e., without UV filters or antioxidants) and incubated with DHR at a depth of ~10 μm was imaged using TPM before and after UV irradiation (20mJ/cm2 UVB-UVA). As Figure 1 illustrates, the ability of TPM to visualize the generation of ROS at different skin depths is one of its greatest advantages. This depth corresponds to the lower SC and is confirmed by the appearance of nucleated cells of the stratum granulosum at 12–15 μm (data not shown). A rainbow scale is used to graphically identify the fluorescence intensity, where blue and red indicate the lowest and maximum fluorescence signals, respectively. As can be seen by comparing the two images, post-UV irradiation causes the fluorescence intensity to increase in the sample, compared with the pre-UV image, signaling the formation of ROS. An absence of obvious cell structure is common in two-photon fluorescence images of the heterogeneous SC.
Similarly, four tissues treated with the four SPF formulations, with and without antioxidants, and each tissue were imaged (30 x 30 μm) at a depth of ~10 μm from the skin surface. Figure 2 shows the TPF images of the four tissues with or without antioxidants after UV irradiationf (20mJ/cm2 UVB-UVA). Each image represents the average intensity of all 40+ images taken for the formulation tested. Formulations contained either UV filters only (Figures 2a–d) or UV filters and antioxidants (Figures 2e–h). All formulations showed a reduction in fluorescence from the skin, compared with the placebo. No difference in fluorescence intensity was detected for the SPF 70 formulation with or without antioxidants; however, antioxidants reduced the R123 fluorescence (ROS levels) for SPF 4, 15 and 50 formulations. In addition, pre-UV data was collected on samples to determine the amount of background fluorescence to be subtracted from the post-UV data using Equation 1. Two pre-UV tissues were imaged per formulation, and at least five areas (30 x 30 μm) were imaged per tissue.
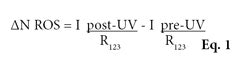
Data Analysis
The average R123 fluorescence intensity was assumed to be proportional to the density of ROS.5–6, 12 The average fluorescence intensity of each image was recorded and each formulation was tested on four tissues, where 10 areas were imaged for each tissue post-UV irradiation. The average fluorescence intensity (I post-UV/R123) was calculated from these 40 images.
To account for background autofluorescence, pre-UV experiments were conducted on the placebo containing no UV filters or antioxidants and the SPF 70 formulation with the highest concentration of UV filters and without antioxidants. Both the placebo and SPF 70 formulations resulted in an identical pre-UV fluorescence signal, indicating that the UV filters and antioxidants did not contribute a signal above the level of autofluorescence emitted by the skin. Therefore, any change in fluorescence intensity could be attributed to the generation of R123 by the reaction with ROS. The average fluorescence intensity was calculated for these samples (I pre-UV/R123) and the average fluorescence intensity pre-UV was subtracted from the average fluorescence intensity post-UV, again as shown in Equation 1.
To calculate the fraction of ROS (fROS) generated by 1 MED UVB-UVA radiation, Equation 2 was used.

In this equation, the control sample ROS generation was equal to skin to which the cream base alone was applied. This emphasized that fROS is a useful value that represents the ability of a formulation to affect ROS levels in the epidermis. It differs from the Radical Skin/Sun Protection Factor (RSF) that uses electron spin resonance (ESR) and a nitroxyl radical probe on skin biopsies containing both the epidermis and dermis.13 Specifically, the absence of dermis in the samples used makes the comparison of the RSF with fROS values impossible. UV irradiation of collagen in dermal tissue generates primary and secondary free radicals in the collagen matrix, which would significantly increase the ROS signal compared to the epidermis-only samples tested.14
In addition, the two methods use different ROS probes that most likely react with different ROS. For example, DHR used in TPM imaging is known to react with singlet oxygen and hydroxyl radical12 but it may not react with all ROS generated. In either technique, the amount of ROS sensitized may actually be greater than what is detected. While determination of the absolute concentration of ROS in live tissue remains a difficult problem, it is assumed that fROS provides an accurate description of the relative ability of a formulation to affect UV-induced ROS levels in the epidermis.
Results and Discussion
It is well-documented that sunscreens reduce erythema. However, their ability to also reduce oxidative stress caused by ROS in UV-exposed skin has only recently been researched.15–28 Studies using ESR to detect and quantify free radicals in UV-irradiated skin ex vivo have shown that sunscreens provide radical protection factors substantially below their labeled SPF value, and that protection against UV-induced free radical formation appears to correlate more with UVA protection than with SPF values.15–17
Through their ability to scavenge ROS, antioxidants with or without sunscreens have been found to provide extra benefits against several different types of skin damage, including: lipid peroxidation, Langerhans cell depletion, cytokeratin 15 induction, epidermal thickening, upregulation of matrix metalloproteinase-1 and matrix metalloproteinase-9 and immune supression.18, 19, 21-28 Similarly, it has previously been shown that the addition of bioconvertible antioxidants to a sunscreen lowered UV-induced ROS levels, compared with the sunscreen alone.20
All of this data points to the need for sun protection products that contain both UV filters and antioxidants. However, in order to maximize the efficacy of both materials, an understanding of the relationship between UV attenuation and antioxidants is required. The data presented here improves upon a previous report where some aspects of the methodology needed refinement.20 In the present report, a solar simulator provides full-spectrum UV irradiation of the skin, an easily consumer-obtainable UV dose of 1 MED is used, and a series of sunscreens with increasing SPF and multiple UV filters was created to represent commercial grade products.
As Figure 2 shows, all formulations reduced the amount of R123 fluorescence relative to the placebo in Figure 1b. Figures 1 and 2 use an intensity scale based upon the fluorescence intensity of the SPF 4 samples; note that if a lower intensity scale were used, the SPF 4 images would be off the scale. Thus, the additional antioxidant effect on the fluorescence intensity for the higher SPF formulations is difficult to see in Figure 2. Figure 3 redisplays the Figure 2image data for SPF 15, 50 and 70 at a lower intensity scale to show the difference in fluorescence intensity between the +/- antioxidant samples. Antioxidants significantly decreased the R123 fluorescence signal for the SPF 4, 15 and 50 formulations but not for the SPF 70 formula.
The results presented here emphasize the importance of adding efficacious antioxidants to sunscreens. While the sunscreens without antioxidants steadily quenched ROS formation as SPF increased, the addition of antioxidants suppressed ROS formation further by 1.7-fold for SPF 4, and 2.4-fold for SPF 15 and 50 formulas. At the highest concentration of UV filters (SPF 70), where little UV penetrates through the sunscreen film at the skin surface, the presence of antioxidants had no statistically distinguishable effect upon the amount of ROS generated, as shown in Figure 4.
Interestingly, Figure 4 also illustrates that even a formula with an SPF of 4, using UVB filters, and with or without antioxidants, provided significant protection against ROS formation. Moreover, SPF 4 and antioxidants provided equal ROS protection to that afforded by SPF 15 without antioxidants, whereas SPF 15 and antioxidants quenched ROS more effectively than SPF 50 without antioxidants. These results show that although higher SPFs may provide greater erythemal protection, they do not necessarily provide greater ROS protection than lower SPF formulas containing efficacious levels of antioxidants. It may also be possible that adding higher concentrations or additional antioxidants to lower SPFs would suppress ROS levels even further. Another interesting finding is that upon exposure to a relatively low UVB-UVA dose of 1 MED through sunscreen-protected skin, a significant amount of ROS is detected in the lower SC. Even the highest SPF tested (SPF 70), which blocks 98.6% of erythemal UV radiation, still led to the formation of ~17% ROS in this experiment. These results are consistent with studies by Flober-Muller et al., who recently reported that sunscreens are crucial at reducing ROS levels, providing a first line of defense against UV-induced ROS. The researchers also reported that the addition of antioxidants must be at effective concentrations to enhance ROS protection further.29 Although some ROS may potentially play important biological roles in the skin, reducing the extra burden of ROS generated during UV exposures may have significant benefits to help maintain skin’s health and appearance throughout life.
Therefore, although traditional sunscreens provide effective protection against the acute symptoms of erythema, they do not provide similarly effective protection against UV-induced ROS in the lower SC. As demonstrated here, supplementation of sunscreens with efficacious antioxidants represents an effective strategy to minimize ROS levels in UV-exposed skin.
Acknowledgements: The experiments in this paper were performed at the Laboratory for Fluorescence Dynamics (LFD) at the University of California, Irvine (UCI). The LFD is supported jointly by the National Center for Research Resources of the National Institutes of Health (PHS 5 P41-RR003155) and UCI. This blind study was sponsored by Merck Consumer Care, and Hanson is a paid consultant for Merck Consumer Care.
References
Send e-mail to [email protected] or [email protected].
1. CL Hexsel and HW Lim, Photoprotection, in Preventive Dermatology, RA Norman ed, Springer-Verlag: London (2010) pp 81–91
2. JF Fisher et al, Mechanisms of photoaging and chronological skin aging, Arch Dermatol 138 1462–1470 (2002)
3. S Pinnell, Cutaneous photodamage, oxidative stress and topical AOX protection, J Am Acad Dermatol 48 1–19 (2003)
4. G Rhie et al, Aging- and photoaging-dependent changes of enzymic and nonenzymic antioxidants in the epidermis and dermis of human skin in vivo, J Invest Dermatol 117 (5) 1212–1217 (2001)
5. KM Hanson and CJ Bardeen, Sunscreen enhancement of UV-induced ROS in skin, Free Radical Biology and Medicine 41 1205–1212 (2006)
6. KM Hanson and CJ Bardeen, Application of nonlinear optical microscopy for imaging skin, Photochemistry and Photobiology 85 33–44 (2009)
7. JP Crow, JS Beckman and JM McCord, Sensitivity of the essential zinc-thiolate moiety of yeast alcohol dehydrogenase to hypochlorite and peroxynitrite, Biochemistry 34 3544–3552 (1995)
8. LM Henderson and JB Chappell, DHR 123: A fluorescent probe for superoxide generation, Eur J Biochm 217 973 (1993)
9. NW Kooy, JA Royall, H Ischiropoulos and JS Beckman, Peroxynitrite-mediated oxidation of DHR 123, Free Radical Biology and Medicine 16 149–156 (1994)
10. JA Royall and H Ischiropoulos, Evaluation of 2’,7’-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells, Arch Biochem Biophys 302 348–355 (1993)
11. BR Master, PTC So and E Gratton, Mutliphoton excitation fluorescence microscopy and spectroscopy of in vivo human skin, Biophysical Journal 72 2405–2412 (1997)
12. KM Hanson and RM Clegg, Observation and quantification of UV-induced ROS in ex vivo human skin, Photochem Photobio 76 57–63 (2002)
13. T Herrling, K Jung, E Chatelain and M Langenauer, Radical skin/sun protection factor RSF—Protection against UV-induced free radicals, SÖFW 132 24–28 (2006)
14. N Metreveli, L Namicheishvili, K Jariashvili, E Chikvaidze and G Mrevlishvili, Microcalorimetric and ESR study of the UV irradiation on collagen, Biofizika 51 29–32 (2006)
15. R Haywood, P Wardman, R Sanders and C Linge, Sunscreens inadequately protect against ultraviolet-A-induced free radicals in skin: Implications for skin aging and melanoma? J Invest Derm 121 862–868 (Oct 2003)
16. U Osterwalder, K Jung, M Seifert and T Herrling, Importance of UVA sun protection: A comparative analysis of different quality control methods, SÖFW 135 (9) 2–13 (2009)
17. K Jung, M Seifert, T Herrling and J Fuchs, UV-generated free radicals in skin: Their prevention by sunscreens and their induction by self-tanning agents, Spectrochim Acta Part A 69 1423–1428 (2008)
18. C Oresajo, M Yatskayer, A Galdi, P Folstis and S Pillai, Complementary effects of AOX and sunscreens in reducing UV-induced skin damage as demonstrated by skin biomarker expression, J Cosm Las Therap 12 157–162 (2010)
19. Y Wu et al, Antioxidants add protection to a broadspectrum sunscreen, Clinical and Exp Dermatol 1–10 (2010)
20. KM Hanson and RM Clegg, Bioconvertible vitamin antioxidants improve sunscreen photoprotection against UV induced ROS, J Cosm Sci 54 589–598 (2003)
21. D Darr et al, Topical vitamin C protects porcine skin from UV radiation-induced damage, Br J Dermatol 127 247–253 (1992)
22. C Elmets, D Singh, K Tubesing, M Matsui, S Katiyar and H Mukhtar, Cutaneous photoprotection from UV injury by green tea polyphenols, J Am Acad Dermatol 44 25–32 (2001)
23. H Gensler and M Magdaleno, Topical vitamin E inhibition of immunosuppression and tumorigenesis induced by UV irradiation, Nutr Cancer 15 97–110 (1997)
24. MT Lopez-Torres et al, Topical application of a-tocopherol modulates the AOX network and diminishes UV-induced oxidative damage in murine skin, Br J Dermatol 138 207–215 (1998)
25. T Nakamura, SR Pinnell, D Darr, I Kurimot, S Itami and K Yoshikawa, Vitamin C: Abrogates, the deleterious effects of UVB radiation on cutaneous immunity by a mechanism that does not depend on TNF-a , J Invest Dermatol 109 20–24 (1997)
26. DI Roshchupkin, M Pistsov and A Potapenko, Inhibition of ultraviolet light induced erythema by AOX, Arch Dermatol Res 266 91–94 (1979)
27. D Steenvoorden and VH Beijersbergen, Protection against UV-induced systemic immunosuppression I mice by a singlet topical application of the AOX Vitamins C and E, Int J Radiation Biol 75 747–755 (1999)
28. K Yuen and GM Halliday, Alpha-tocopherol, an inhibitor of epidermal lipid peroxidation, prevents UV radiation from suppressing the skin immune system, Photochem Photobio 65 587–592 (1997)
29. H Flober-Muller, S Champ, C Kandzia, K Jung, M Seifert and T Herling, Strategy for efficient prevention from photoaging, SÖFW 134 23–32 (2008)