The current building blocks of permanent hair colorant were identified in the 1860s. In 1863, German chemist August Wilhelm von Hofmann, Ph.D., discovered p-phenylene diamine (PPD), a key synthetic dye.1 Then in 1867, London chemist E.H. Thiellay and Parisian hairdresser Leon Hugot demonstrated the use of hydrogen peroxide to lighten hair. By 1907, the first commercially available permanent hair colorant was created by Eugene Schueller2 and by 1956, the products were available in an in-home kit using a one-step process for lightening and color formation.
Since that time, new hair dyes have been added to the color palette but the basic chemistry remains unchanged. The color transformation these products can deliver, i.e., covering unwanted gray hair or changing hair to a totally new shade, has grown this category to US $7+ billion worldwide, according to internal research, with markets continuing to expand. While the transformational color benefits are compelling, women also recognize that these products ultimately lead to hair damage. Internal consumer testing has shown that women surveyed about what causes hair damage choose permanent colorants among their top answers, along with use of high temperature implements such as flat irons.3
This article explores the main changes to hair after regular use of permanent hair colorants and how these changes impact noticeable hair properties such as feel, styling, breakage, etc. It then considers several strategies to reduce the damage caused during hair coloring, or minimize the impact of damage post-coloring. All of these interventions have a positive impact on women, allowing them to achieve the color they desire but with minimal consequences to hair health.
How Hair Changes During Coloring
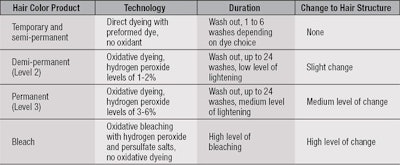
Hair color and bleach products are divided into four different categories depending on the technology used, as shown in Table 1. These chemistries give different levels of color transformation in terms of both the level of lightening of the underlying hair color and amount of synthetic color delivered; and how long this transformation lasts. Importantly, the chemistry used also determines the severity of damage to the hair fibers.
This review is focused on permanent hair color, or Level 3 products. These products contain two bottles that are mixed together immediately before use. One bottle contains hydrogen peroxide, typically either 6% or 9% active, at a pH of 2-4, and one bottle contains dye precursors and an alkalizer, typically ammonia or ethanolamine, at a pH of 10-11. The final mixed pH is 10. The mixed product is applied to dry hair and left for 25-40 min before rinsing. Figure 1 shows a schematic of the two processes that occur to form the final color.
The first step involves the bleaching of hair melanin by perhydroxyl anions, which are formed by increasing the pH of hydrogen peroxide to pH 10. This lightens the underlying color of hair. The second step is the diffusion of dye precursors into hair and the coupling of these dyes, which is activated by hydrogen peroxide, to form an extended chromophore, which is the new color. A range of shades can be achieved by altering the degree of lightening and changing hydrogen peroxide levels, or by changing the amount and combination of dye precursors. Many hair color products contain four to eight different dye precursor structures that can couple together to give a range of shades including blondes, reds, browns and blacks.
The same oxidative chemistry responsible for creating the final hair color is also responsible for changing hair properties. There are two key reactive species formed by hydrogen peroxide at pH 10: the perhydroxyl anion, as shown in Equation 1; and hydroxyl radicals formed catalytically in the presence of redox active metals—mainly copper and to a lesser extent, iron, as shown in Equation 2.
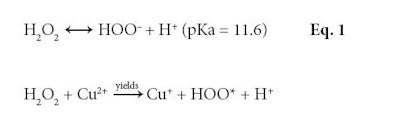 Strategies to Repair and Minimize Oxidative Damage
Strategies to Repair and Minimize Oxidative DamageMany strategies to reduce the damage caused by oxidative hair dyes have been in the area of surface materials. These return the hydrophilic surface properties of hair, caused by removal of the F-Layer lipid, closer to the hydrophobic state of uncolored hair. Such materials include silicones, quats and even F-Layer lipid itself.13
Silicones are the most commonly used materials to deliver this surface benefit. They generally are modified to have some polarity so they are attracted to the negative charge of colored hair. Amodimethicones, for example, incorporate amino groups or silicone quats that include quaternary charged groups.14 By restoring hydrophobic surface properties, both wet and dry combing forces are decreased and flyaways caused by static are improved.15 These materials also protect hair from breakage by reducing tangling, and many provide a degree of wash-fade protection, slowing the rate whereby the formed dye is removed from hair during washing.16 The mechanism of action for these materials is likely related to the formation of a hydrophobic surface coating, which moderates the diffusion of hydrophilic dyes out of hair.
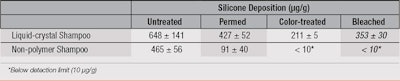
An alternative strategy is to alter the surface hydrophobicity of hair by adding a material such as a polymer that deposits on hair and then further enhances the deposition of a non-polar silicone, such as polydimethylsiloxanes (PDMS). An example of this strategy would be the incorporation of a high charge density cationic polymer, poly(diallyldimethyl ammonium chloride) (poly(DADMAC)), combined with anionic surfactants in a shampoo.17 This combination forms a liquid crystal structure that on dilution, deposits from the shampoo onto hair to: provide “slip planes” along the hair surface for wet conditioning purposes; and form a hydrophobic layer that changes the surface energy of the fibers. This increased hydrophobicity allows for enhanced silicone deposition on previously colored or bleached hair, as shown in Table 3.
Summary
In summary, the hair color category is likely to continue growing, and consumers will seek the desired transformations of covering gray or achieving a shade different from their own. With the current oxidative chemistry, which delivers both lightening and color-formation, there will also be associated changes to hair structure signifying poor hair health. These include loss of shine, poor feel, breakage, etc. However, there are opportunities to reduce the damage that occurs during coloring and prevent further damage by protecting hair after coloring.
References
- S Handa, R Mahajan and D De, Contact dermatitis to hair dye: An update, Indian J Dermatol Venereol Leprol 78 583–90 (2012)
- S Pointer, The Artifice of Beauty: A History and Practical Guide to Perfume and Cosmetics, The History Press, Stroud, England (May 1, 2005)
- G internal report, global data (2013)
- S Godfrey, W Staite, P Bowtell and J Marsh, Metals in female scalp hair globally and its impact on perceived hair health, Int J Cosmet Sci 35(3) 264-271 (2013)
- CR Robbins, The cell membrane complex: Three related but different cellular cohesion components of mammalian hair fibers, J Cosmet Sci 60 437-465 (2009)
- A Vaynberg, M Stuart and XF Wu, Differential wetting characterization of fibers, J Cosmet Sci 63(1) 33-41 (2012)
- RL McMullen, G Zhang and T Gillece, Quantifying hair shape and damage induced during reshaping of hair, J Cosmet Sci 66 379-409 (2015)
- JM Marsh, RM Dahlgren, C Clarke, J Stonehouse and C Nunn, A new oxidant for hair coloring, J Cosmet Sci 60 205-215 (2009)
- AD Bailey, G Zhang and BP Murphy, Comparison of damage to human hair fibers caused by monoethanolamine and ammonia based hair colorants, J Cosmet Sci 65 1-9 (2014)
- JM Marsh et al, Preserving fiber health: Reducing oxidative stress throughout the life of the hair fiber, Int J Cosmet Sci (37) supplement S2 16-24 (2015)
- JM Marsh et al, Role of copper in photochemical damage to hair, Int J Cosmet Sci 36(1) 32-38 (2014)
- JM Marsh et al, Advanced hair damage model from ultra-violet radiation in the presence of copper, Int J Cosmet Sci 37 532-541 (2015)
- M Okamoto, N Tanji, T Habe, S Inoue, S Tokunaga and H Tamamachi, ToF-SIMS characterization of the lipid layer on the hair surface. II: Effect of the 18-MEA lipid layer on surface hydrophobicity, Surf Interface Anal 43(1-2) 298-301 (2011)
- S Herrwerth, I Ulrich-Brehm, U Kortemeier, P Winter, M Ferenz and B Gruening, Silicone quaternium-22: New silicone technology for premium hair conditioning with additional benefits, SÖFW 135(6) 11-12, 14-18 (2009)
- HM Haake, S Marten, W Seipel and W Eisfeld, Hair breakage—How to measure and counteract, J Cosmet Sci 60 143-151 (2009)
- A Schlosser, Silicones used in permanent and semi-permanent hair dyes to reduce the fading and color change process of dyed hair occurred by wash-out or UV radiation, J Cosmet Sci 55(suppl) S123-131 (2004)
- MA Brown, TA Hutchins, CJ Gamsky, MS Wagner, SH Page and JM Marsh, Liquid crystal colloidal structures for increased silicone deposition efficiency on color-treated hair, Int J Cosmet Sci 32(3) 193-203 (2010)