The antimicrobial properties of silver ions have been known since ancient times, and several studies have explored the mechanisms of action that make them effective against such a broad range of microorganisms.1 This includes Gram-positive and Gram-negative bacteria,2, 3 fungi and yeasts,3 and viruses.4–6 Silver ions work in a number of ways, most noticeably by disrupting essential cell transport, i.e., the movement of nutrients, metabolites and waste in, out and around the cell; protein synthesis;7 and reproduction by interfering with the microorganism’s DNA and RNA.8, 9 This effectively starves or suffocates the microorganism, preventing its replication and resulting in inert stasis or death.
However, some microorganisms have a greater tolerance of silver than others, and even show a degree of resistance. Silver ions are non-selective and as noted, affect the performance of a number of critical physical functions within microorganisms. Therefore, in order to become resistant to silver ions, an untreated microorganism would need to mutate several critical functions simultaneously in a single generation through spontaneous mutation. The likelihood of this is very low;10–13 to date, the emergence of any silver-sensitive pathogen made resistant from the clinical use of silver has not been reported.
Further, one can conclude from the vast body of scientific work on this topic1, 4, 5, 14–17 that it is indeed the silver ion that is the antimicrobial agent and not metallic silver itself or silver salts, oxides or complexes. There are always some ions present in equilibrium with the stable silver form;9 the more stable the form, the lower its associated ionic concentration and, in the case of silver, the lower its antimicrobial activity. It is only the ions in equilibrium with these stable forms that are active.1 No lasting disinfection can therefore take place unless there is a sufficient and continuous replenishment of the active silver ions. This is illustrated by the use of silver nitrate compresses on wounds, where treatment requires frequent application of the solution—as often as every two hours—to sustain an effect.
Silver ions, however, are reactive and short-lived because they form complexes with other molecules.9, 18, 19 As such, the antimicrobial potency of silver can only truly be unlocked with a suitable method of delivering free silver ions in a controlled and sustained manner. This author’s interest to incorporate the antimicrobial potency of silver into medical devices, primarily for the wound care and urology markets, led to exploring the potential of water-soluble phosphate glassesa, also known as phosphate polymers, as a controlled delivery platform for the silver ions.
Phosphate Polymers
The vitreous sodium, calcium phosphate systems investigated herein are prepared in a fusion process at more than 1,000°C to produce a material that is water-soluble, dissolving completely in any aqueous environment and leaving no residue. Any degradation products are naturally occurring and abundant in the human body and the environment, making it an intrinsically safe material.14, 15, 20–22 Interestingly, during production of the phosphate polymer, bioactive metals such as silver can be incorporated. Silver ions effectively become trapped in the glassy structure and when the material comes into contact with water, the oxygen bridges holding the structure together hydrolyze. These bioactive ions are then released into the environment as discrete, free ions—potentially in high concentrations. Unlike systems such as nano silver, these phosphate polymers release every single atom of silver as an effective silver ion in a controlled, pre-ordained delivery profile.14–16 They do not rely on massive surface area and the vagaries of the aqueous environment. As such, they are inherently efficient, thus reducing environmental impact.
The rate of release of the ions is primarily determined by the solution rate of the phosphate polymer carrier, which in turn is determined by its chemical formulation. To illustrate the range of solution rates,23 a 1.0-mm bead made with the slowest-dissolving phosphate polymer, having a solution rate of 0.0006 mg/cm2/hr, would last just under 25 years when dissolved in deionized water at 37.5°C. A bead of the same size made with the fastest-dissolving phosphate polymer material, having a solution rate of approx. 2,500 mg/cm2/hr, would last about 10 min under the same conditions; i.e., the fastest material dissolves just over four million times faster than the slowest. The material can be formulated to give virtually any solution rate within this range.
Other factors that affect the rate of release include the concentration of the bioactive ions; the shape, size and environment into which the material is placed; the transport of dissolved product; extremes of pH, etc. Therefore, the formulation is developed to give the required delivery profile for the application and environment in which the material will be used. Using this technology, the delivery of high-potency, long-lasting antimicrobial activity based on controlled silver ion release has been made possible in several commercial medical device products. This same technology also has the potential to add value to personal care products, delivering antimicrobial functionality, e.g., with silver, or other skin benefits through incorporation of other bioactive metal ions such as zinc.
The present paper investigates the antimicrobial performance of a silver-releasing phosphate polymer formulation incorporated into a simple bar soap product. In the first study, suspension test data versus five pathogens are presented. This demonstrates the speed and efficacy of the silver-based anti- microbial. The second study explores the longevity of the antimicrobial effect. These studies also illustrate more generally the potential of these materials as platforms for the controlled, sustained release of bioactive metal ions in cosmetic products.
Study 1: Materials and Methods
As noted, to test the speed and efficacy of the silver-based antimicrobial, a bar soap product was prepared using a standard vegetable-based formulation but containing sodium, calcium silver phosphate at concentrations of 0.1%, 0.2%, 0.5%, 1.0%, 2.0%, 5.0% and 10%. The sodium, calcium silver phosphate added to the soap was formulated to dissolve rapidly to compensate for the short contact time with soap while it is in use,23 and to ensure the optimum and continuous release rate of silver ions.16, 23 The formulation of the soap product included, among other ingredients, a moisturizer and vitamin E to permit frequent use without damaging the skin. Frequent use can cause skin to become red and sore, which happens with some antibacterial washes, cleansers and alcohol gels.
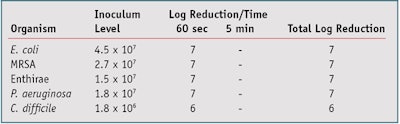
To evaluate the bactericidal activity of these samples, a method based on BS EN 1276 was employed.24 This suspension test is used to assess the bactericidal activity of chemical disinfectants and antiseptics used in food, domestic and institutional areas. This is an absolute rather than comparative test; as such there is no control and a product can be deemed an antimicrobial if a reduction of ≥ log 5 of all test organisms is achieved after 5 min of exposure. Readers should note that while it may have been instructive to include a sample without the silver-containing active, the test protocol does not require this. Further, despite the observation that all soaps are antimicrobial to some extent, the prospect of the soap base achieving effects of the magnitude observed seems implausible.
The test product was suspended at a concentration of 5% using simulated dirty conditions by the addition of 3.0g/L bovine albumin (final concentration) and the procedure was carried out at a control temperature of 20°C. The test organisms used were: Escherichia coli ATCC 8739, Methycillin-resistant Staphylococcus aureus NCTC 12493, Enterococcus hirae ATCC 10541, Pseudomonas aeruginosa ATCC 9027 and Clostridium dificille NCIMB 10666. The culture medium used for this test was a combination of Tryptone Soya Agar and Columbia Agar, with the addition of defibrinated horse blood.
Results: Bactericidal Activity
The results presented in Table 1 indicate that the soap formulations comply with the test pass criteria stipulated in the BS EN 1276 suspension method, and were able to reduce the inoculum level of the pathogens listed by a minimum log of 5 after 5 min of exposure. The results obtained with active concentrations of 0.1%, 0.2%, 1.0%, 2.0%, 5.0% and 10% were identical to those presented for 0.5%.
Study 2: Materials and Methods
To explore the longevity of the antimicrobial effect, two bacterial strains particularly relevant to hand hygiene were used: S. aureus and S. marascens. The experiment protocol involved washing a pigskin model with soap,25 rinsing with clean water, inoculating with an emulsion of the chosen organisms, swabbing and recovering residual organisms, and re-inoculating and repeating after one hour and two hours.
Results: Duration of Effects
Figure 1 shows that more than 99% of the added bacteria were absent after the first test, and that after the second and third tests, more than 99% of S. aureus and S. marascens were absent. After the first test, S. marascens was only inoculated to 50% the original level.
Conclusions
While making no specific claims for being effective against certain pathogens, the BS EN 1276 test highlights the efficacy of the silver-releasing phosphate polymer as an antimicrobial active ingredient against several notable microorganisms. The second study indicates that antibacterial activity continues for several hours after washing. Current alcohol-based sanitizers and disinfectant soaps clean the skin but leave it immediately ready for recolonization. The longevity testing demonstrates that the sodium, calcium silver phosphate continues working on the skin after use, thereby reducing the buildup of viable pathogens between washes.
This is a simple approach to incorporating sophisticated metal ion delivery cost effectively into bar soap, liquid soap, sanitizers, balms, toothpaste, shampoos and soap wafers. The phosphate polymers can deliver antimicrobial ions like silver, copper and zinc or combinations of metal ions. Additional uses could include neutralizing odors, i.e., by binding with thiol groups; providing genuine antioxidant activity with certain metals and metal ion combinations, e.g., SOD mimetics; and clearing warts, verrucas, eczema and other topical conditions, for which there is much anecdotal evidence of silver’s ability. Other elements with beneficial skin properties could also be included, such as selenium for dry scalp conditions. In addition, the particle size of the phosphate polymer could be adjusted to provide effective exfoliation—and since they are totally water–soluble, the particles would leave no residue and pose no downstream issues for waste water management.
References
- VN Golubovich, The toxic effect of ionic silver on various groups of microorganisms, Mikrobiologiya 43 922–924 (1974)
- K Kawahara, K Tsuruda, M Morishita and M Uchida, Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions, Dental Materials 16, 6 452–455 (2000)
- Giltech Ltd., Internal microbiological studies data; more than 100 evaluations of silver, copper, zinc and other metal ion studies against a group of 17 core screened organisms (gm+ve, gm-Ve pathogenic bacteria and two yeasts) and numerous one-off organisms; also studies on 16 pathogenic fungi covering the main pathogenic fungal families (1988–2012)
- HH Lara, EN Garza-Treviño, L Ixtepan-Turrent and DK Singh, Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds, J Nanobiotechnology 3 9:30 (Aug 2011)
- DX Xiang, QChen, LPang and CL Zheng, Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro, J Virol Methods 178(1-2):137–142 (Dec 2011)
- S Galdiero, A Falanga, M Vitiello, M Cantisani, V Marra and M Galdiero, Silver nanoparticles as potential antiviral agents, Molecules 16(10) 8894–8918 (Oct 24, 2011)
- RL Davies and SF Etris, The development and functions of silver in water purification and disease control, Catalysis Today 36 107–114 (1997)
- SL Percival, PG Bowler and D Russell, Bacterial resistance to silver in wound care, J Hospital Infection 60 1–7 (2005)
- JP Guggenbichler, M Boswald, S Lugauer and T Krall, A new technology of microdispersed silver in polyurethane induces antimicrobial activity in central venous catheters, Infection 27 16–23 (1999)
- A Gupta and S Silver, Silver as a biocide: Will resistance become a problem? Nature Biotechnology 16 10, 888 (1998)
- EJ Lowbury, Infection associated with burns, Postgraduate Medical Journal 48 560, 338–341 (1972)
- EJ Lowbury, Problems of resistance in open wounds and burns, in: Mouton, RP et al, eds, The Rational Choice of Antibacterial Agents, London, Kluwer Harrap Handbook (1975)
- A Lansdown and A Williams, Bacterial resistance to silver-based antibiotics, Nursing Times; 103 9, 48–49 (2007)
- CF Drake and WM Allen, The use of controlled release glass for the controlled delivery of bioactive materials, Biochemical Society Transactions 13 516–520 (1985)
- T Gilchrist, DM Healy and C Drake, Controlled silver-releasing polymers and their potential for urinary tract infection control, Biomaterials 12 76–78 (1991)
- AG Avent, CN Carpenter, JD Smith, DM Healy and T Gilchrist, The dissolution of silver–sodium–calcium-phosphate glasses for the control of urinary tract infections, J Non-crystalline Solids 328 31–39 (2003)
- C Lok et al, Silver nanoparticles: Partial oxidation and antibacterial activities, J Biological Inorganic Chem 12, 4 527–534 (2007)
- BS Fox, MK Beyer and VE Bondybey, Coordination chemistry of silver cations, J Amer Chem Soc 124 (45) 13613–13623 (2002)
- BS Fox, MK Beyer and VE Bondybey, Coordination chemistry of silver cations, J Amer Chem 124 (45) 13613–13623 (2002)
- CN Carpenter, The chemistry of a novel silver controlled release glass, doctoral thesis, University of Sussex (1996)
- J Burnie and T Gilchrist, Controlled release glass (CRG)—A bew biomaterial, Ceramics in Surgery, P Vincenzini, ed, Elsevier Science Publishers, Amsterdam (1983) pp 169–176
- J Burnie, SRI Duff, CF Drake, NGL Harding, AJ Malcolm and T Gilchrist, Controlled release glasses (CRG) for biomedical uses, Biomaterials 2 244–246 (1981)
- Giltech Ltd., Internal analytical data (1988–2012)
- British Standards Institution BS EN 1276:2009, available at https://knowledge.bsigroup.com/products/chemical-disinfectants-and-antiseptics-quantitative-suspension-test-for-the-evaluation-of-bactericidal-activity-of-chemical-disinfectants-and-antiseptics-used-in-food-industrial-domestic-and-institutional-areas-test-method-and-requirements-1/standard (Accessed Nov 1, 2012)
- Microbiological experiments performed for the Standard Soap Company, Ashby de la Zouch, UK by Microbiological Solutions, Manchester UK (Feb 2007)